Generation of the knockout cell line (ZAK and RACK1 knockout)
HEK293T RACK1-knockout cells were generated by CRISPR–Cas9 (refs. 51,52). The single guide RNA (sgRNA; ZAK target sequence: TGTATGGTTATGGAACCGAG; RACK1 target sequence ACTGCGGGGTAGTAGCGATCTGG) was subcloned into the pX330 plasmid51. HEK293T cells (American Type Culture Collection CRL-3216) were transfected with 1.5 µg of plasmid using Lipofectamine 3000 (L3000075, Thermo) according to the manufacturer’s instructions. After 2 days, cells were collected and plated on 96-well plates (3603, Corning) by limiting dilution. Colonies were confirmed for ZAK or RACK1 deletion by immunoblotting and sequencing.
Tissue culture
HEK293T cells were maintained using Dulbecco’s modified eagle medium (DMEM; 11995073, Thermo) supplemented with 10% FBS (A3160502, Thermo Fisher) and passed using trypsin-EDTA (0.25%) and phenol red (25200114, Thermo). Cells were seeded at 2 × 106 (15 cm; CLS430599, Millipore Sigma), 1.5 × 106 (10 cm; CLS430167, Corning) or 3 × 105 cells per well (six well; 3516, Fisher). At 24 h, cells were transfected using Lipofectamine 3000 Transfection Reagent (L3000075, Thermo) according to the manufacturer’s instructions. Twenty-four hours post-transfection, cells were treated and lysed (approximately 70% confluency). Medium was changed 1–2 h before drug treatment and/or lysis. ANS (A9789, Sigma) was added directly to the media (untreated = DMSO (D12345, Thermo); ANS collision dose = 0.38 µM final concentration). ANS stock solutions were 94.2 mM (25 mg ml−1) in DMSO. Unless noted, all ANS treatments were done for 15 min. For the protein stabilization experiments, medium was supplemented with 2 µM MLN4924 (B1036, ApexBio) or 2 µM nilotonib (A8232, ApexBio) from the time of transfection to time of harvesting (24 h). To end any treatment, medium was aspirated and cells were quickly washed with ice-cold PBS (10010-049, Thermo; 8 ml for 10 cm; 2 ml for six well) and then the lysis buffer (50 mM HEPES pH 7.5, 100 mM KOAc, 5% glycerol (G33-4, Fisher), 0.5% Triton X-100 (T9284, Millipore Sigma), 15 mM Mg(OAc)2, 1× Halt protease + phosphatase inhibitor cocktail (78445, Thermo Fisher) and Turbo DNase I (80 units; AM2239, Thermo)) was directly added to plates and the cells were collected by scraping. Plates (10 cm) were lysed in 200 µl lysis buffer; six-well dishes were lysed in 100 µl lysis buffer. Lysates were kept on ice and clarified at 8,500g for 5 min. Clarified lysates were flash frozen in N2 and stored at −80 °C.
RNA knockdowns
Cells were seeded at 1 × 106 cells (10 cm) and 7.5 × 104 (six well). After 24 h, cells were treated with siRNA (50 µM stocks, 50 nM final concentration) using Lipofectamine RNAiMAX Transfection Reagent (13778150, Thermo) according to the manufacturer’s instructions. After 24 h, the medium was changed. Seventy-two hours post-siRNA transfection, cells were treated and lysed according to the above lysis protocol.
Sucrose gradients
Preparative (12 ml) sucrose gradients were made using 10× gradient buffer (250 mM HEPES pH 7.5, 1 M KOAc and 50 mM Mg(OAc)2) to make final gradients with sucrose buffer (1× gradient buffer, 10% or 50% sucrose (60% sucrose stock), 1 mM TCEP (TCEP25, Gold-Bio) and SuperaseIN (200 units)). Approximately 25–50 µg RNA (quantified by qubit HS) was loaded on gradients in 200–300 µl final volume. The Beckman Coulter Ultracentrifuge and Beckman SW41 swinging bucket rotor were used for centrifugation. For regular gradients (10–50% sucrose), spins were done at 274,000g for 1 h 45 min at 4 °C. Ten fractions were collected and absorbance at 260 nm (A260) was measured using the Biocomp Piston Gradient Fractionator. Trichloroacetic acid (TCA; T3699, Millipore Sigma) was added to each fraction (10% final concentration). Samples were frozen at −20 °C overnight. The TCA precipitation protocol followed.
Analytical (200 µl) gradients were made by stacking 40 µl of 50%, 40%, 30%, 20% and 10% sucrose buffer in 250 µl tubes (343775, Beckman). Approximately 1–2 µg RNA (quantified by qubit or normalized by bicinchoninic acid (BCA)) was loaded in 10 µl final volume on a 10–50% 200 µl gradient. The Beckman Coulter Tabletop Centrifuge (CTZ24D006, Optima MAX) and TLS55 rotor were used for centrifugation. Spins were done at 214,000g for 22 min at 4 °C. Ten 20 µl fractions were taken and added directly to 7 µl of 4× loading buffer. Of each fraction, 8 µl was run on 4–20% TGX 26-well gel (5671095, Bio-Rad).
Immunoblotting
Concentration-normalized samples were generated using total protein quantification (BCA assay; 23225, Thermo Fisher) and were then diluted in 4× loading buffer (8% sodium dodecyl sulfate (SDS), 40% glycerol, 0.4 mM bromophenol blue and 40 mM Tris-Cl pH 6.8) and boiled at 95 °C for 10 min. Approximately 5 µg of protein was loaded into 4–20% Criterion TGX polyacrylamide gels and run in 1× Tris-glycine running buffer (1610732, Bio-Rad) at 150 V for 1 h. Proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Trans-Blot Turbo RTA Midi 0.2 µm PVDF Transfer Kit 1704273) for 10 min at 2.5 A. Membranes were blocked with 5% non-fat milk (blocking buffer; sc-2325, Santa Cruz Biotech) in Tris-buffered saline with Tween 20 (TBS-T) for 1 h at room temperature. All primary antibody incubations were done overnight at 4 °C in blocking buffer. After three 10-min TBS-T washes, secondary antibodies diluted in blocking buffer were incubated for 1 h at room temperature. Three 10-min washes with TBS-T followed secondary incubation. All incubations and washes were performed with gentle rocking. Blots were visualized using SuperSignal West Pico PLUS (34580×4, Thermo) and/or West Femto Maximum (34095, Thermo) chemiluminescent substrates and the Bio-Rad ChemiDoc imaging system.
Phos-tag SDS–PAGE
Concentration-normalized samples were diluted in 4× loading buffer and boiled as described for immunoblotting and then loaded on 8% SDS–PAGE (10.7 µM Phos-tag acrylamide (AAL-107S1, Wako) and 21.3 µM MnCl2). Samples were run in 1× Tris, glycine and SDS running buffer (125 V for 2.5 h). An EDTA-free pre-stained protein marker (F4005, Apex Bio) was used. Two 10-min washes in 1× transfer buffer (25 mM Tris, 192 mM glycine and 10% v/v methanol) supplemented with 1 mM EDTA (AM9260G, Thermo) were followed by two 10-min washes in 1× transfer buffer without EDTA. Samples were transferred to PVDF membranes (1620177, Bio-Rad) overnight at 35 V (room temperature). Blocking and antibody incubation and visualization are described in the section ‘Immunoblotting’.
TCA precipitations
Sucrose fractions in 10% TCA were thawed on ice and centrifuged at 21,000g at 4 °C for 30 min. The sucrose and TCA were aspirated off and the remaining pellet was washed with 500 µl acetone (A18P-4, Thermo) and spun at 21,000g at 4 °C for 10 min. The wash step was repeated and then the pellets were dried using a vacuum evaporator for 5 min. The dried pellets were resuspended in 4× loading buffer (40–60 µl total; 10 µl loaded on gel).
Co-immunoprecipitation of ZAK dimers
ZAK-knockout HEK293T cells were transfected with either FLAG-tagged or haemagglutinin-tagged ZAK constructs (24-h expression) and then treated and lysed from six-well plates (see the section ‘Tissue culture’; lysis buffer: 50 mM HEPES pH 7.5, 100 mM KOAc, 5% glycerol, 1% digitonin (D-180-1, Gold Bio), 5 mM Mg(OAc)2, Halt protease + phosphatase inhibitor cocktail (100×) and Turbo DNase I (80 units)) according to the above tissue culture protocols (six well, 100 µl lysis buffer). After scraping, the cells were incubated on ice for 30 min before clarification and flash freezing. On the day of the experiment, cells were thawed on ice and normalized using A260 as measured by nanodrop (13-400-529, Thermo Scientific). MNase buffer (0.2×; B0247S, NEB) with 1 mM CaCl2 and 500 U MNase (M0247S, NEB) were added to normalized lysates and the reaction was incubated at 22 °C for 30 min. The samples were moved to ice and 2 mM EGTA (50-997-744, Fisher) was added. For the co-immunoprecipitations, 90 µl of sample was added to 2 µl washed anti-FLAG M2 magnetic affinity resin (M8823, Millipore) and incubated for 90 min with rotation at 4 °C. The samples were washed three times with 100 µl wash buffer (50 mM HEPES pH 7.5, 100 mM KOAc, 5% glycerol, 0.1% digitonin, 5 mM Mg(OAc)2 and Halt protease + phosphatase inhibitor (100×)) for 5-min incubation on nutator at 4 °C. The protein was eluted (elution buffer: wash buffer plus 400 μg ml−1 3×FLAG peptide) with two 10 µl elutions (30 min incubation on rocker at 4 °C). Samples were added to 4× loading buffer and immunoblots were performed according to the above described methods.
CLIP sample preparation
CLIP-seq samples were prepared as previously described53. Two 10-cm plates per construct of HEK293T ZAK-knockout cells (pcDNA3.1-FLAG-ZAK_T161/S165A, pFN24K_3×FLAG-ZAK, pFN24K_3×FLAG-ZAK_T161/S165A and pFN24K_3×FLAG-ZAK_1-649) were transfected in addition to two 10-cm mock no DNA control plates. After 24 h of expression and 15 min of ANS treatment, cells were crosslinked at 254 nM UV, lysed, clarified and RNAseI digested (AM2295, Invitrogen). Immunoprecipitation was performed overnight at 4 °C using 13 µl of anti-FLAG M2 affinity resin (A2220, Millipore). Following immunoprecipitation, samples were washed, dephosphorylated with FastAP enzyme (EF0654, Thermo) and T4 PNK (M0201L, NEB) and the 3′ RNA adapter was ligated (M0437M, NEB). A small amount of sample was run on a Criterion XT 4–12% Bis-Tris gel and transferred to PVDF to perform a diagnostic western blot probing for ZAK expression and size. The remaining sample was run on a Criterion XT 4–12% Bis-Tris gel (3450124, Bio-Rad) and transferred to a nitrocellulose membrane and a region was cut corresponding to the ZAK protein size to plus approximately 75 kDa. Membrane pieces were digested with proteinase K (P8107S, NEB). RNA was purified on a clean and concentrator column and reverse transcribed with SuperScript III (18080044, Thermo). The RNA and free primer were digested and the RT-DNA purified with MyOne Silane beads (37002D, Thermo) and the 5′ DNA adapter was ligated (M0437M, NEB). Following cleanup, a pilot quantitative PCR was performed. Samples were amplified for the determined number of PCR cycles, gel extracted for products corresponding to 175–35 bp and submitted for next-generation paired-end sequencing with 2 × 150-bp read length.
CLIP analysis
All code for CLIP-seq analysis has been published on GitHub (https://github.com/jakesaba/2025_ZAK). In brief, unique molecular identifiers were appended to each paired-end read using umi_tools extract54 and trimmed using trim_galore (https://github.com/FelixKrueger/TrimGalore). Reads were aligned using STAR55 to the GRCH38 genome containing a single ribosomal DNA (chrR), originally generated by the Paralkar laboratory56. Aligned reads were sorted and indexed using samtools57 and deduplicated using umi_tools dedup.
For mapping coverage to 18S rRNA, bam files were imported into R, coverage was normalized to library size and then mean-scale normalized across the 18S region. Mean-scaled coverage over 18S was then normalized to the coverage of the ZAK_1–649 truncation1 at each position. To avoid dividing by 0, a pseudocount corresponding to the 0.1 percentile signal was applied to the coverage of the ZAK_1–649 sample at all positions. To reduce noise, nucleotide positions corresponding to less than 3% of the cumulative CLIP-seq coverage signal were removed and their fold enrichment was set equal to 1. Plots were smoothed using a rolling average with a window size of 10.
For genome-wide analysis of CLIP-seq peaks, a similar approach was used with a few exceptions. First, no mean-scale normalization was applied and coverage was normalized to the ZAK-knockout sample. A global pseudocount of 5 was applied and cumulative signal less than 3% of the cumulative CLIP-seq coverage at each gene locus was again removed. CLIP peaks with average reads per million of more than 10 and satisfying a more than twofold enrichment over a window size of more than 20 compared with the ZAK knockout were called. Significance was determined using a one-sided Poisson test. For significant peaks, a false discovery rate was assigned using the Benjamini–Hochberg procedure. For each gene, only the canonical transcript was used.
For metagene analysis, we aligned CLIP-seq coverage data to standardized transcript regions (5′ untranslated region (UTR), the coding sequence (CDS) and 3′ UTR). For each gene, only the canonical transcript was used, and only transcripts with a CDS length of at least 300 nucleotides were retained. For each transcript, the 5′ UTR, CDS and 3′ UTR were separately scaled to 100 positions, and coverage values were linearly interpolated to create a fixed-length alignment across all genes. These were concatenated to produce a ‘metagene’ axis of 300 standardized positions (0–100 for 5′ UTR, 100–200 for CDS and 200–300 for 3′ UTR). To account for background signal, the metagene profile of each condition was normalized to a ZAK-knockout control profile, computed as the ratio of the mean signal to the ZAK-knockout signal at each metagene position. For visualization, smoothed profiles (rolling average, window size = 5) were plotted with region boundaries clearly marked.
Bacterial expression and purification of ZAK RBR
For ZAK RBR (C terminus 100 amino acids) protein expression, the sequence was cloned into pGEX backbone with a N-terminal GST tag. The plasmid was transformed into BL21-competent cells (C2527I, NEB) and allowed to outgrow overnight at 37 °C. The starter culture was added to a 1-l flask of 2× YT media (31GE58, Grainger), and at optical density at 600 nm of 0.6, protein expression was induced with 1 mM IPTG for 2 h. Bacterial pellets were collected (4,000g for 10 min), flash frozen and stored at −80 °C.
Pellets were thawed on ice in lysis buffer (50 mM Tris pH 8, 150 mM NaCl, 5% glycerol, 1 mM TCEP, 0.2 mM phenylmethylsulfonyl fluoride (PMSF; P7626-25G, Sigma), 1× EDTA-free cOmplete protease inhibitor tablet (5056489001, Sigma), pinch of DNase I (10104159001, Millipore Sigma) and pinch of lysozyme (L6876, Sigma)) to a final volume of 50 ml and dounced on ice until fully resuspended. The lysate was sonicated at 50% amplitude (3 s on; 10 s off; 1 min total) before clarification using the TI45 rotor and spinning at 186,000g for 30 min. After the spin, the supernatant was filtered using 0.45-µM filter (431220, Corning) and loaded onto GSTrap 5 ml column (17513102, Cytiva) using the Cytiva (GE Healthcare) AKTA Pure FPLC system. After binding, the column was washed with wash buffer 1 (50 mM Tris pH 8, 150 mM NaCl, 1 mM TCEP, 0.2 mM PMSF and 1 protease inhibitor pill) and high-salt wash buffer 2 (50 mM Tris pH 8 and 1 M NaCl). The protein was eluted (elution buffer: 50 mM Tris pH 8, 300 mM NaCl and 10 mM reduced glutathione) and the eluted fractions were pooled and concentrated using Pierce Protein Concentrators PES, 30 K MWCO (88522, Thermo) to 1 ml before size-exclusion chromatography (Cytiva Superdex 75) with SEC buffer (50 mM HEPES pH 7.5, 300 mM KOAc, 5 mM Mg(OAc)2, 5% glycerol and 1 mM TCEP). Protein samples were concentrated, flash frozen and stored at −80 °C until use.
Native pull-downs of ZAK-bound ribosomal complexes for cryo-EM
Expi293F cells (A14527, Thermo Fisher) transiently transfected with the pcDNA3.1-FLAG-ZAK-K45M construct were treated with ANS (0.38 μM) for 15 min and collected in lysis buffer (50 mM HEPES pH 7.5, 150 mM KOAc, 5 mM Mg(OAc)2, 1 mM dithiothreitol, 0.5% NP-40 and EDTA-free protease inhibitor cocktail (Roche)). Cells were homogenized using a dounce homogenizer (DWK Life Science) and clarified by centrifugation at 36,603g for 15 min at 4 °C. The supernatant was treated with Nuclease S7 (20 U ml−1; Sigma-Aldrich) for 15 min at 25 °C. Digested lysates were incubated with Anti-FLAG M2 agarose beads (Sigma-Aldrich) on a rotating wheel for 3 h at 4 °C. Beads were transferred to a 1-ml Mobicol column (MoBiTec) and washed twice with 10 ml of wash buffer (50 mM HEPES pH 7.5, 150 mM KOAc, 5 mM Mg(OAc)2, 1 mM dithiothreitol and 0.01% NP-40). Complexes were eluted in elution buffer (20 mM HEPES pH 7.5, 150 mM KOAc, 5 mM Mg(OAc)2, 1 mM dithiothreitol, 0.05% Nikkol and 300 ng µl−1 FLAG peptide (Sigma-Aldrich)) for 1 h at 4 °C.
The same purification protocol as described above was also used for the Strep–ZAK(T161A/165A) and FLAG–ZAK(K45M/K394D) pull-downs as well as for the FLAG–ZAK(K45M) pull-down performed without previous challenging cells with ANS (see also Extended Data Fig. 1b).
In vitro binding assays and reconstitutions of ZAK RBR–ribosome complexes
Human ribosomal subunits and 80S monosomes were purified from Expi293F cells. Cells were lysed in lysis buffer (50 mM HEPES (pH 7.5), 150 mM KOAc, 5 mM Mg(OAc)2, 0.5% NP-40, 1 mM dithiothreitol, 1 mM PMSF and EDTA-free protease inhibitors). Lysates were clarified by centrifugation at 36,603g for 15 min, loaded onto 10–50% sucrose gradients and spun at 284,600g for 3.5 h at 4 °C using a SW40Ti rotor (Beckman Coulter). Gradients were fractionated into 500-µl fractions to separate 40S and 60S ribosomal subunits from 80S monosomes. 40S, 60S and 80S fractions were pooled and pelleted through a sucrose cushion using a TLA110 rotor (Beckman Coulter) at 460,800g for 45 min at 4 °C, then resuspended in binding buffer (50 mM HEPES pH 7.5, 150 mM KOAc, 5 mM Mg(OAc)2, 1 mM dithiothreitol and 0.01% NP-40).
For the in vitro binding assay, the GST-3C-tagged ZAK RBR protein (see above) was incubated with either purified ribosomal subunits or monosomes (25 pmol each) for 60 min at 4 °C. Reactions were diluted with 360 µl binding buffer and transferred to 1-ml Mobicol columns (MoBiTec) containing 20 µl glutathione Sepharose 4 fast flow resin (Cytiva) and incubated for 60 min at 4 °C. Beads were washed three times with binding buffer (1 × 800 µl, 2 × 500 µl). Bound complexes were eluted with binding buffer containing 25 mM reduced L-glutathione (Sigma-Aldrich) for 60 min at 4 °C. Samples were analysed on a 12% polyacrylamide gel (Invitrogen) and stained with Der Blaue Jonas (German Research Products).
Electron microscopy and image processing
For all cryo-EM samples, grids were prepared and images were processed the same way. All samples were crosslinked with 0.02% (v/v) glutaraldehyde on ice for 20 min. Reactions were quenched by addition of Tris-OAc to a final concentration of 25 mM. Of each sample, 3.5 μl (approximately 4–8 A260 per ml) was applied to Quantifoil R3/3 holey carbon grids with 2-nm continuous carbon coating, blotted for 3 s and then plunge frozen in liquid ethane using a Vitrobot Mark IV (Thermo Fisher Scientific). Data collections were performed at 300 keV using a Titan Krios microscope equipped with a Falcon 4i direct electron detector and a SelectrisX imaging filter using EPU software (3.7; all Thermo Fisher Scientific) at a pixel size of 0.727 Å. Dose-fractioned movies were collected in a defocus range from −0.5 to −3.5 μm and with a total dose of 40 e− Å−2, fractionated in 40 frames to obtain a total dose of 1 e− Å−2 per frame. Gain correction, movie alignment and summation of movie frames were performed using MotionCor2 (v1.4.0)58. Contrast transfer function parameters were estimated using CTFFIND4 (v4.1.13)59.
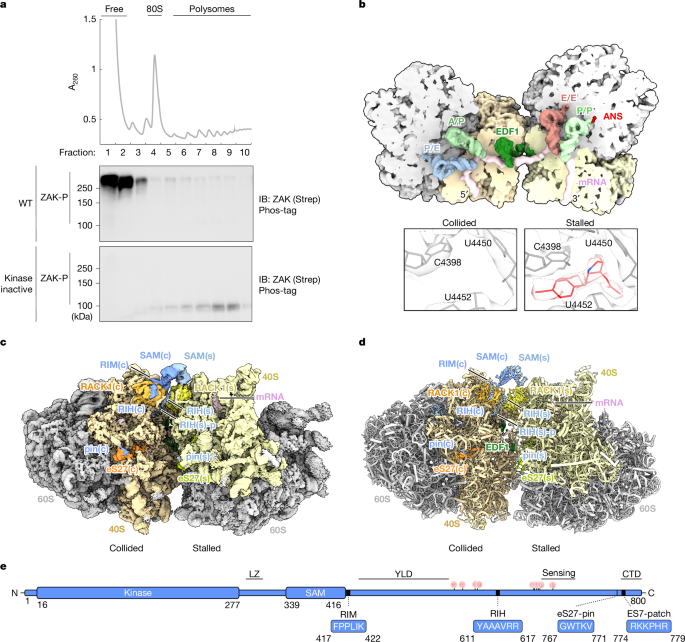
Structures of ZAK–disome complexes were obtained from native pull-downs after treatment with ANS using either the FLAG-tagged ZAK(K45M) mutant or the ZAK(T161A/165A) mutant.
From the combined datasets, 2,246,220 particles were automatically picked from a total of 95,813 micrographs in RELION (v5.0 beta)60. After 2D classification in CryoSPARC (v4.6.0)61, 1,347,732 80S ribosomal particles were selected and 3D classified in RELION. This yielded 80S classes with strong density for neighbours, indicative of stable disomes, or with no or only weak extra density for neighbours. 80S with no neighbour density represented either 80S with mRNA and tRNAs in hybrid state (A/P and P/E) or POST state (P/P and E/E), or 80S bound to eEF2 (and SERBP1). Disome classes (80S classes with strong neighbour density) occurred either with a neighbour at the mRNA exit site or at the mRNA entry site, defining them as stalled and collided 80S, respectively. Both stalled and collided 80S were found in POST and in hybrid states, and each of those disome classes displayed additional density at RACK1, accounting for the ZAK SAM dimer. The two most abundant classes (POST-state stalled 80S and hybrid-state collided 80S) were further processed. They were classified with a soft mask focusing on the RACK1 region where extra density for ZAK was found, revealing subclasses with the ZAK SAM dimer varying in flexibility. Classes displaying strong SAM density were joined and refined to an overall resolution of 2.3 Å for both the stalled 80S and the collided 80S, and then subjected to local refinement in CryoSPARC. Local resolution was determined for the RACK1 SAM regions to be between below 3 Å (for the RACK1–RIM interaction) and between 5 Å and 8 Å for the globular SAM domain. Finally, for a sub-dataset, two maps for the entire ZAK–disome complex were generated by extending the box size of both stalled and collided 80S, respectively, and centring the disome density. Both maps showed low-resolution extra density adjacent to the SAM dimer, probably accounting for the leucine zipper–kinase domain (LZ–KD) dimer. The disome obtained from extending the stalled 80S was locally refined focusing on the RACK1 SAM region, yielding a clear density for the SAM dimer at a local resolution between 6 Å and 11 Å and served as consensus disome map. The disome obtained from extending the colliding 80S was used for fitting the LZ–KD dimer.
The composite map for the ZAK–disome was assembled by first fitting the individually refined stalled and collided 80S and the locally refined maps into the 6 Å resolution disome consensus map and then using the ‘vop max’ tool in UCSF Chimera X (v1.9)62 to join the individual maps (Extended Data Fig. 2).
The sample obtained from the native FLAG–ZAK(K45M) pull-down without ANS treatment was processed as described above. Here, however, 3D classification of 325,370 particles picked from a total of 13,154 micrographs showed no classes indicative for stable disomes. Instead, the main classes represented two classes of translating 80S, one with tRNAs in hybrid state (A/P and P/E) and one with tRNAs in POST state (P/P and E/E), as well as 80S with a tRNA in the E site and bound to eEF2 and SERBP1, indicative of hibernating ribosomes38,39. The hybrid state translating and the hibernating 80S classes were first refined followed by local refinement focusing on either the entire 40S subunit (2.3 Å for the translating and 2.7 Å for the hibernating 40S) or the RACK1 region (2.7 Å for the translating and 3.0 Å for the hibernating 40S head; Extended Data Fig. 3a).
For the sample obtained from the FLAG–ZAK(K45M/K394D) pull-down, 3D classification of 629,553 particles picked from a total of 56,746 micrographs yielded similar 80S classes as described for the pull-downs using kinase-inactive ZAK mutants described above. Among them were classes representing disomes as well as hibernating (with eEF2/SERBP1 and E tRNA) and translating (hybrid and POST state) 80S. The classes representing stalled (with P/P and E/E tRNAs) and collided (with A/P and P/E tRNAs) 80S were refined followed by local refinement focusing on the RACK1 SAM region. Local resolution was determined for this region to be below 3 Å close to RACK1 and to between 5 Å and 15 Å for peripheral regions. We observed density for one SAM globular domain emerging from RACK1 of the collided ribosome, whereas on RACK1 of the stalled one is occupied by SERBP1 and the ZAK–RIH (Extended Data Fig. 8).
For the reconstituted ZAK–RBR–40S sample (obtained from the in vitro binding assay), 873,389 particles were picked from a total of 19,851 micrographs. 3D classification yielded 629,046 particles of 40S that were further refined, followed by local refinement focusing on either the head (2.1 Å) or the eS27 (2.3 Å) region (Extended Data Fig. 5d).
Model building and refinement
The disome model was generated by rigid-body fitting known 80S monosome structures into the cryo-EM density. For the stalled 80S, the ribosome structure (Protein Data Bank (PDB) ID 8GLP)30 representing a POST-state 80S with mRNA and P-site tRNA, bound ANS was used; for the collided 80S ribosome, the structure of the human ribosome in the hybrid PRE state (PDB ID 6Y57)29 served as a template. E-site tRNA model from the human disome (PDB ID 7QVP)34 was used and fitted into density on the stalled 80S map. The tips of ES6c (690–740) and ES6b (741–800) were built based on AlphaFold3 prediction of 18S rRNA fitting into low-pass-filtered density on the collided 80S and the stable disome reconstructions (Extended Data Fig. 2). The mRNA model on the collided 80S from PDB ID 6Y57 was changed from 46-UUU-48 to AUG and 49-UUU-51 to UUC.
For the EDF1 C-terminal part (residues 24–145) on the collided 80S, the existing model from (PDB 6ZVH)21 was fitted. For the N-terminal part (residues 7–14) on the stalled 80S, the AlphaFold3 prediction for the interaction of EDF1 with eS26 was used for fitting (Extended Data Fig. 5f,g). A rod-like density near uS4 on the collided 80S was identified as the C terminus of eS1 (254–264) and was modelled based on AlphaFold3, predicting an interaction between eS1 residues 231–264 with uS4. These models were processed by manual real-space refinement in WinCoot (v0.9.8.93)63 and merged into a disome model followed by real-space refinement in Phenix (v1.20.1-4487)64.
AlphaFold2 multimer models of ZAK full length and RACK1 revealed two interacting regions: one is ZAK 611–617 (RIH) intercalating between blade 5 and blade 6 of RACK1, and the second is ZAK 417–422 (RIM) stretching across RACK1 blade 2 and blade 3. This model served as a template to match extra densities identified at the RACK1 (Extended Data Fig. 5a–e).
To adjust RIM and RIH, respectively, we then generated AlphaFold3 models of ZAK 325–425 and RACK1, and models of ZAK 600–631 and RACK1. The resulting models were fitted into the corresponding density with only minor adjustments in Coot (Extended Data Fig. 5a–e).
The main density emerging from both RACK1 proteins at the disome interface corresponds to two SAM (residues 328–416) domains extending from the N terminus of RIM, prompting us to model a SAM dimer using AlphaFold2 multimer. Among the predicted dimer models, only the one representing the asymmetric head-to-tail interface fitted our density as a rigid body. Here manual adjustment was required only for helix α5 (393–416) on both SAM domains to fit into the clearly resolved rod-like density extending from the RACK1-bound RIM peptide. An additional rod-like extra density packed against the SAM domain on the stalled 80S was interpreted as an α-helix formed by residues 568–583 of ZAK (‘helix’), based on the AlphaFold database (https://alphafold.ebi.ac.uk/). The pin was identified by running AlphaFold3 predictions of the RBR region (701–800) and eS27, and then fitted into the corresponding density followed by minor adjustment in Coot.
A model for the C terminus of SERBP1 (SERBP1-C; residues 393–408) bound to RACK1 together with the ZAK RIH was generated with AlphaFold3 and adjusted using the 2.9 Å resolution map of the locally refined RACK1 from the hybrid-state translating ribosome (Extended Data Fig. 3a) followed by Phenix refinement.
AlphaFold3 models for the LZ–KD dimer (1–330) were docked into an additional globular density on the entire ZAK–disome complex when low-pass filtered to approximately 30 Å (Extended Data Fig. 2).
The ZAK model and disome model were later merged and further refined in Phenix. Model statistics were calculated using the MOLPROBITY implementation in PHENIX65, and can be found in Extended Data Table 1.
All structural figures were prepared using UCSF Chimera X (v1.9)62.
Statistics and reproducibility
Unless otherwise noted, all biochemical experiments and cell-based assays were repeated a minimum of two times (in part or in whole) and the two independent replicates showed similar results.
Antibodies used in study
The primary antibodies used were: rabbit anti-eS24 (ab196652, Abcam; 1:1,000); mouse anti-FLAG (A8592, Sigma; 1:5,000); rabbit anti-haemagglutinin (3724, Cell Signaling; 1:1,000); mouse anti-JNK1 (3708, Cell Signaling; 1:1,000; ‘total JNK’); rabbit anti-phospho-SAPK/JNK (4668S, Cell Signaling; 1:1,000; ‘JNK-phospho’); rabbit anti-RACK1 (5432S, Cell Signaling; 1:1,000); rabbit anti-SERBP1 (NBP1-85660, Novus; 1:1,000); mouse anti-STREP (71591-3, Sigma; 1:5,000); mouse anti-vinculin (sc-73614, Santa Cruz; 1:2,000); and rabbit anti-ZAK (A301-993A, Fortis; 1:1,000).
The secondary antibodies used were: anti-mouse (7076S, Cell Signaling; 1:5,000) and anti-rabbit (7074S, Cell Signaling; 1:5,000).
Oligonucleotides used in study
Non-targeting (scramble) siRNA (D-001810-01-20, Horizon Dharmacon) and SERBP1-targeting siRNA (L-020528-01-0005, Horizon Dharmacon) were used.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.