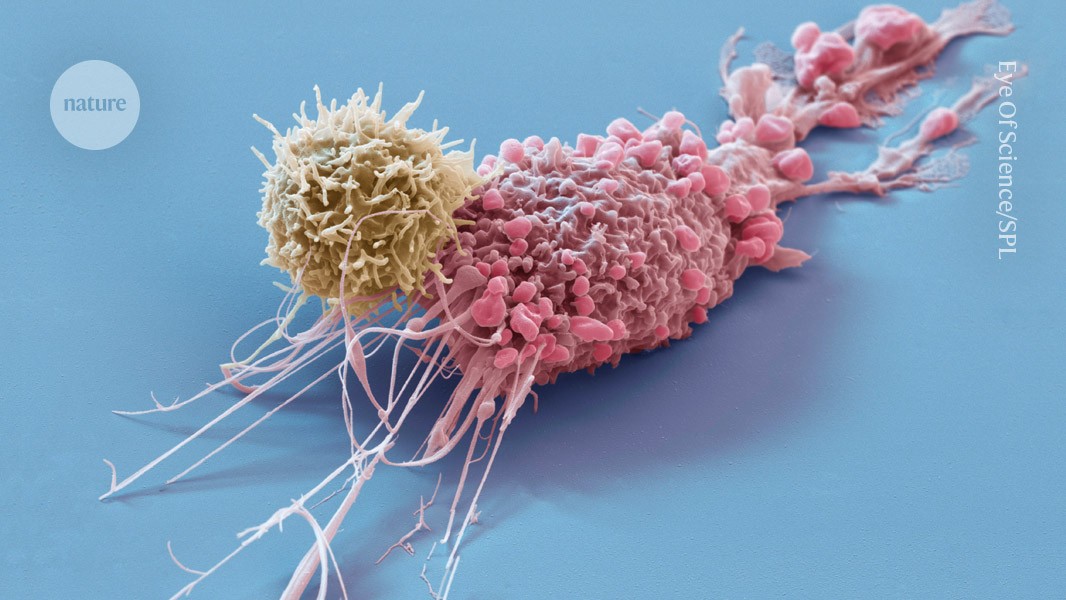
Engineered immune cells, called CAR T cells (yellow), have revolutionized treatment for some tumours (pink) and show promise for treating autoimmune conditions. Credit: Eye Of Science/Science Photo Library
One woman and two men with severe autoimmune conditions have gone into remission after being treated with bioengineered and CRISPR-modified immune cells1. The three individuals from China are the first people with autoimmune disorders to be treated with engineered immune cells created from donor cells, rather than ones collected from their own bodies. This advance is the first step towards mass production of such therapies.
One of the recipients, Mr. Gong, a 57-year-old man from Shanghai who has systemic sclerosis, which affects connective tissue and can result in skin stiffening and organ damage, says that three days after receiving the therapy he felt his skin loosen and he could start moving his fingers and opening his mouth again. Two weeks later, he returned to his office job. “I feel very good,” he says, more than a year after receiving the treatment.
Engineered immune cells, called chimeric antigen receptor (CAR) T cells, have shown great promise in treating blood cancers — half a dozen products are approved in the United States — and potential for treating autoimmune conditions such as lupus and multiple scleroris, in which rogue immune cells release autoantibodies that attack the body’s own tissue. But the therapy typically relies on a person’s own immune cells, and this personalization makes it expensive and time consuming.
That’s why researchers have started creating CAR T therapies from donated immune cells. If successful, they would allow pharmaceutical companies to scale up manufacturing, potentially slashing costs and production times. Instead of making one treatment for one person, therapies for more than a hundred people could be made from one donor’s cells, says Lin Xin, an immunologist at Tsinghua University in Beijing. Donor-derived CAR T cells have been used to treat people with cancers, but with limited success so far2.
Autoimmune diseases
The trial, led by Xu Huji, a rheumatologist at Naval Medical University in Shanghai, is the first to report results for autoimmune diseases. They were published in Cell last month. More than six months after receiving the treatment, the recipients remained in remission. Another two dozen individuals have received the donor-derived treatment, and a slightly modified product, says Xu. The results have been largely positive, he says.
“The clinical outcomes are phenomenal,” says Lin, who is leading a separate trial using donor-derived CAR T cells to treat lupus.
The success and safety of the therapy look promising but still need to be demonstrated in many more people before researchers can draw conclusions about its broad application, says Christina Bergmann, a rheumatologist at the University Hospital Erlangen in Germany.
But if it does succeed in more people over a longer time frame, it “could prove paradigm shifting”, says Daniel Baker, an immunologist at the University of Pennsylvania in Philadelphia. More than 80 autoimmune diseases are linked to malfunctioning immune cells.
Healthy donor
CAR-T-cell therapy typically involves extracting immune cells known as T cells from the person being treated. The cells are embellished with CAR proteins that target B cells and are then re-infused into the person’s body.
The process for creating CAR T cells from donated immune cells is similar. Xu and his colleagues extracted T cells from a 21-year-old woman and studded them with CARs that recognize CD19, a receptor found on the surface of B cells. They used the CRISPR–Cas9 gene-editing tool to knock out five genes in the T cells, to prevent both the grafted cells from attacking the host’s body and the host’s immune system from attacking the donor cells.
The first person to receive the treatment, in May 2023, was a 42-year-old woman with a type of autoimmune myopathy, which targets skeletal muscle tissue, resulting in weakness and fatigue. Mr Gong, and another man aged 45, had an aggressive form of sclerosis. They started their treatments in June and August 2023.
Once injected into the hosts, the CAR T cells got to work. They multiplied and targeted and destroyed all the B cells — including pathogenic cells linked to the autoimmune conditions. The bioengineered T cells survived for weeks in the recipients before largely vanishing. Eventually, new healthy B cells returned, but no pathogenic ones did. A similar response has been observed in people with autoimmune conditions who received CAR T cells derived from their own cells3.
‘Complete remission’
Two months after the treatment, the researchers say the woman achieved complete remission, and maintained that status at her six-month follow-up. Baker says that although the woman showed clear clinical improvements, he would be more cautious about calling it complete remission given the short assessment time. The woman’s autoantibodies had dropped to undetectable levels, and her muscle strength and mobility had improved dramatically.
The two men also saw significant improvements in their symptoms — including the reversal of scar-tissue formation — and declines in autoantibody levels.
None of the individuals experienced an extreme inflammatory reaction known as cytokine-release syndrome, which has been observed in some people with cancer who have received CAR-T therapy, and they didn’t show evidence of the graft attacking the host. But the researchers are still trying to determine if over time, the host rejects the graft.
One key safety concern observed in some people who have received CAR-T-cell therapy to treat cancer is the emergence of new tumours, although researchers are still investigating whether they are linked to the therapy. Baker says it’s too early to know whether people with autoimmune conditions who are treated with donor-derived CAR T cells will face this risk. “Only time will tell.“
The big question now, Baker says, is whether the same approach will work in more people, and how durable the effects will be. “Will these patients stay symptom-free for years?”