Unravelling how the billions of interacting neurons in the human brain conjure consciousness is one of the greatest challenges in twenty-first-century science. Over the past decade, large, well-funded initiatives, including in the United States, Europe and China, have been launched to unlock the mysteries of cognitive function — mental processes such as memory, language, perception and problem-solving — by coming at it from all angles.
For the millions of people around the world who will develop an incurable or treatment-resistant brain disorder this year, the need to better understand cognitive function and dysfunction is pressing, says Christopher Rozell, a computational neuroengineer at Georgia Institute of Technology in Atlanta. Rozell co-leads a multidisciplinary team that is developing technology-based therapies for depression, the leading cause of ill health and disability worldwide. “Globally, more than 300 million people will have a major depressive episode this year — and that’s just one neurological disorder subtype,” he says.
Nature Index 2024 Neuroscience
Rozell is exploring a therapy for treatment-resistant depression based on deep-brain stimulation, in which implanted electrodes electrically stimulate specific brain areas to provide long-term symptom relief. The work is funded by the US National Institutes of Health’s (NIH) Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative, a major project launched in 2013, which to date has invested more than US$4 billion across neuroscience research. The BRAIN Initiative’s strategy is to develop tools, and then use these advances to gain a deeper understanding of brain function. According to Rozell, the decade-long investment is beginning to pay off.
In depression treatment, for example, doctors have always had to make subjective clinical judgements and trial-and-error therapy adjustments when trying to manage the condition. But, in 2023, Rozell and his collaborators used new brain-implant and big-data processing technologies to identify changes in brain activity that can indicate a patient’s current clinical state, enabling doctors to adjust treatment in response1. At the end of the six-month trial, 90% of patients showed significant improvement and 70% were in remission or no longer depressed. BRAIN Initiative funding was key. “We work with clinicians and engineers in teams with a breadth of expertise that would have been very difficult to imagine under conventional funding programmes,” Rozell says. “Every week now, you see large, interdisciplinary teams making incredible advances that would not be happening if it were not for a programme like the BRAIN Initiative.”
Likewise, proponents of the Human Brain Project (HBP), one of the largest research endeavours ever funded by the European Union (EU), which spent €600 million (US$668 million) over ten years before its completion in 2023, point to several advances. New brain-implant technologies that could restore partial vision in certain forms of blindness and brain-like ‘neuromorphic’ computer chips for more sophisticated artificial intelligence (AI) are important outcomes.
But concerns remain that core questions in neuroscience have not been addressed by big projects. It’s not clear how cognitive function emerges from patterns of brain activity, for instance, let alone how these processes go awry caused by disease.
And although big-neuroscience funding has increased in China over the past few years, it has been cut significantly in the EU and the United States, threatening the trajectory of brain science advancement.
Uncharted territory
If understanding human brain function is the ‘moonshot’ of neuroscience, we’ll never make it without the right maps, says Rozell. Creating brain atlases, each focused on different structural features, has been a key aim. In late 2023, the BRAIN Initiative’s Cell Census Network (BICCN), a multi-centre effort led by the Allen Institute for Brain Science in Seattle, Washington, produced the most detailed map yet of the cells that make up the human brain. Using single-cell genome sequencing — a technique that allows all or part of an individual cell’s genome to be sequenced — the team identified more than 3,000 different cell types in the human brain, many previously undescribed.
BICCN researchers also produced the first complete cellular atlas of a mammalian brain, pinpointing the location and identity of each of the more than 32 million cells in a mouse brain2. When the team launched the project 10 years ago, it was unclear whether this was even feasible, says Allen Institute director, Hongkui Zeng, who led the work. But the rapid development and scaling-up of single-cell genomic technology has revolutionized the field.
“Previously, the brain was just an unknown number of faceless cells,” says Zeng. “Now, we have the molecular identities for specific cells in specific brain regions, and we can start to label each cell type and see what they do.”
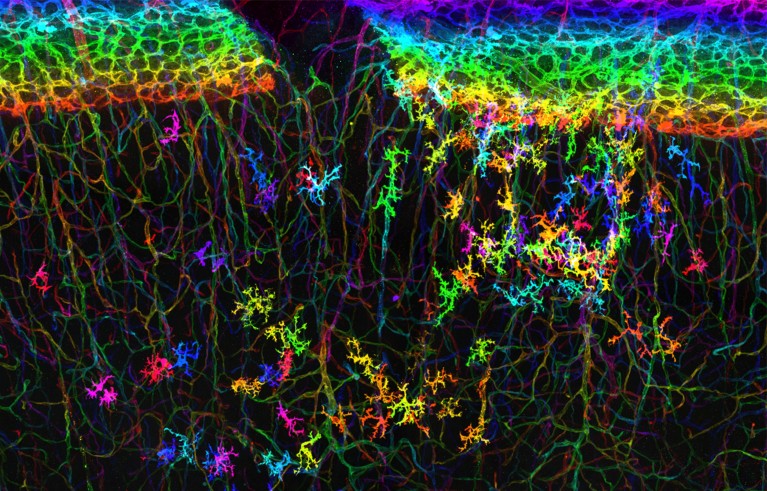
Immune cells in a mouse brain, intertwined with tiny blood vessels, captured for a BRAIN-funded project.Credit: Josephine Liwang, Yongsoo Kim lab/Penn State College of Medicine, PA
BICCN’s open-access brain-cell atlases are an indispensable resource, says Sebastian Seung, a computer scientist and neuroscientist at Princeton University in New Jersey. “To go from mapping the brain as a bunch of regions, to mapping cell types, is a huge jump in precision,” he says. Brain-cell atlases are foundational data supporting Seung’s own research, which focuses on the wiring between brain cells, known as the connectome. Together with cell mapping, new tools in connectomics — including those developed in Seung’s lab with BRAIN funding, which use AI to automate brain-scan image processing — allow scientists to study the brain in ways they’ve never done before.
A different approach was used to build the Human Brain Atlas, the most detailed 3D anatomical map of a human brain yet assembled3. A team led by Katrin Amunts, a neuroscientist at the Jülich Research Centre, a large-scale national facility in the Helmholtz Association of German Research Centres, took a postmortem brain and analysed it, slice by slice, to build the atlas not from the cells up, but from a whole brain down. The Human Brain Atlas forms a core part of EBRAINS, an open-access digital platform that combines tools, services and data generated by the HBP, which has been used by more 10,000 people worldwide.
The platform’s ‘virtual brain’ tool is being used to create personalized patient brain models to guide clinical decision-making in epilepsy, multiple sclerosis, depression and Parkinson’s, and its brain atlases and data are being accessed by researchers in neuroimaging, neurology, AI and basic science. In January, the EBRAINS project won a further €38 million from the European Commission to fund its continued development.
There is an argument that although BRAIN and the HBP did not specifically focus on conceptual questions in neuroscience, the foundational resources that they have provided can help to fill major knowledge gaps that will benefit those working in both basic and applied neuroscience areas. Seung says this is why the BRAIN Initiative’s strategy of prioritizing neuroscience tool development was the right approach. “So much of the study of neuroscience has been limited by the scarcity of data,” he says. “The NIH would normally not necessarily fund technology development, but sometimes to get to important science, we need a technological revolution.”
New model
Still in its early phases, China’s big neuroscience project can benefit from lessons learned by its international counterparts. Conceived in 2013 — closely following the launch of BRAIN and the HBP — the China Brain Project (CBP) began in 2021 with ten-year funding of 12 billion yuan (US$1.66 billion) to advance brain-disease studies and basic neuroscience, as well as brain-inspired technologies and brain–computer interfaces. The project involves more than 500 laboratories across the country, and aims to build on China’s research strengths, including in connectomics and non-human primate animal models, a valuable, but contentious, aspect of neuroscience. “You cannot do invasive experiments in the human brain to understand what’s going on, so animal models are very important,” says Zeng.
The protocols and standards for non-human primate research in China are based on those set by the NIH, but the work is easier to conduct because animal-rights groups don’t protest against animal use in research like they do in the United States, says Muming Poo, scientific director of the Institute of Neuroscience at the Chinese Academy of Sciences in Shanghai, who has led the CBP organizing committee since 2020. “There is a great need in the community for using non-human primate disease models because mouse models for brain disease, especially psychiatric disease, are just not working,” says Poo. He notes the slow global pace of drug development for brain disease, which is mostly based on rodent models, and says non-human primates, as our closest living relatives, should offer better models of the human brain.
Poo’s group is developing a toolbox of genetic-engineering techniques to produce non-human primate models of disease that they hope can be used in drug testing. In late 2023, they reported the first live-born monkey chimaera4, created by taking stem cells from one macaque embryo and adding them to another. The work is a key step towards creating transgenic non-human primate models of human brain diseases, akin to way that transgenic rodent models of disease are currently made.
Another strength that the CBP hopes to build on is China’s vast population, from which researchers can draw on extensive patient cohorts. According to Jialin Zheng, dean of the Tongji University School of Medicine in Shanghai, autism spectrum disorder in children, depression in adults and Alzheimer’s disease in ageing populations are the priority conditions addressed by CBP research.
In parts of the CBP that are related to brain-inspired technology, such as AI and brain–computer interfaces, there is strong competition between institutions in China and abroad, says Poo. But in basic neuroscience and brain medicine, the CBP was specifically designed to complement work conducted by other countries. “We made a strong point of taking the directions that are deficient in the United States and Europe,” such as non-human primate models and large-cohort studies, says Poo. Some of the first internationally collaborative research conducted within the project are now close to publication, he adds. “I think it’s like the global-warming problem — brain disease is an urgent problem shared by all of society, and we should solve it together.”
In many ways, the approaches and priorities of the big-brain projects in the United States, Europe and China complement each other to make the most of international resources and talent. In the United States, for instance, the BRAIN Initiative pooled resources to push technology development, whereas the HBP’s strategy focused on coordinating multidisciplinary research, such as bringing neuroscientists together with computer scientists to develop new treatments. China’s strategy is to use its unique strengths to fill important gaps and expand on them through international collaboration.
There are challenges ahead if researchers want to build on the outputs of the three initiatives. For example, Zheng says it’s going to require coordination between governments to decide how genetic information and biological samples can safely be shared between countries. “Different countries have different regulation in terms of data. How can it be shared more openly? We are dealing with the same diseases, so, how can we work together to address these challenges?”
In addition to restrictions on data sharing, coordination between different data centres is a major issue, says Poo. “It has been difficult to set up a generally agreed, smooth way of data-sharing among many big projects, because each big project has its own data centre,” he says. “We are in international discussions about the data problem, but there is no solution yet.”
There are also concerns about whether long-standing questions around cognitive function can be answered by the kinds of projects being funded by big brain programmes. On the one hand, finding answers will require parallel studies of brain activity at the molecular, anatomical and physiological levels — something that large-scale initiatives are designed to facilitate, says Zeng.
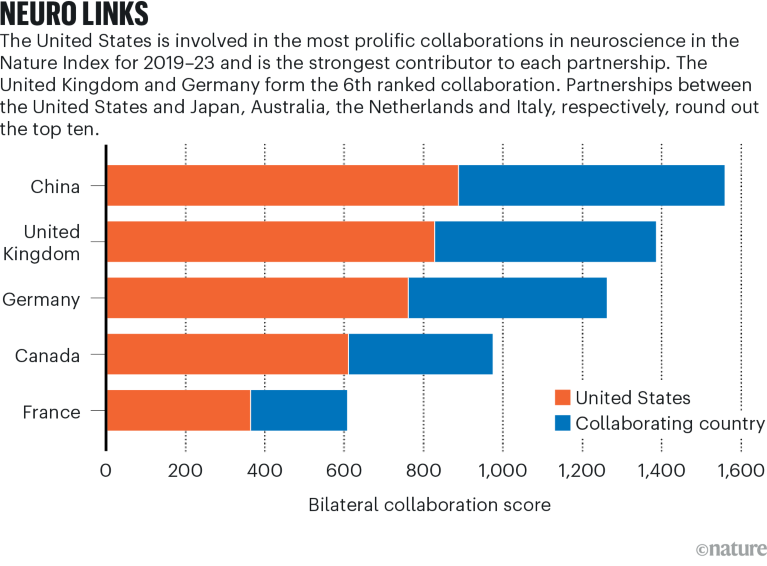
Source: Nature Index
But knowing how to piece this information together to explain cognitive function will require new ideas and hypotheses at a foundational level that none of the big neuroscience projects has yet produced, says Yves Frégnac, emeritus research director in cognitive science at the University of Paris-Saclay in France. “New concepts are not evolving at the same pace as technologies,” he says. “Reading out signs of cognitive activity is very different from understanding the brain.”
For China, the CBP has brought a much-needed injection of cash to a field that has struggled to find funding in the past. Poo says the initiative, which so far seems to be on track to meet its decade-long funding promise, will not only advance neuroscience in highly applied areas, but also in fundamental research. “In other countries, there are avenues of support for basic research in brain science, through organizations such as the US NIH or National Science Foundation — but not in China,” he says.
As the CBP builds momentum, researchers in Europe are trying to regain their footing, a year after the end of the HBP. Raising just over half of the expected €1 billion in funding from the EU and its member states, the HBP feels to many scientists like an opportunity not quite fulfilled, despite the progress made. “This money was needed in the field of brain sciences,” says Frégnac, who wrote an opinion piece on how the initiative could have been done better5. “People talk about €1 billion, US$4 billion, but if you compare it to initiatives in physics, this is peanuts.” NASA’s James Webb Space Telescope, for example, cost $10 billion, and the $1.5 billion annual budget of the European particle-physics laboratory, CERN, dwarfs the HBP’s entire ten-year funding. “If we want to be serious about the brain, we need to put more money in,” says Frégnac, who adds that the possibility of a well-funded follow-up to the HBP looks remote.
The future of BRAIN Initiative-supported research is also unclear. In 2024, as the ten-year pot of funds set aside in 2016 entered its ramp-down phase, a budget cap across all federal spending constrained the US Congress from making up the shortfall. The result was a 40% cut to BRAIN Initiative funding, compared with 2023. Researchers such as Rozell, whose work on treating depression is directly threatened by the cuts, are worried. “We’ve made enormous progress, but this work is not finished — it is not an approved therapy,” says Rozell. With the global economic cost of mental disorders estimated at US$5 trillion, the need for investment is clear, he adds. “To have spent a decade of money, time and expertise to reach a place where we’re starting to see the returns, and then have the threat of these programmes being taken away, it’s enormously concerning.”



