In biomedical science, China still lags behind the United States and Europe when it comes to fundamental research and conducting clinical trials involving investigators and participants from several countries1. But the nation is now a global leader when it comes to drug development and manufacturing. And it is becoming increasingly important in frontier science.
How China is vying to attract the world’s top scientific talent
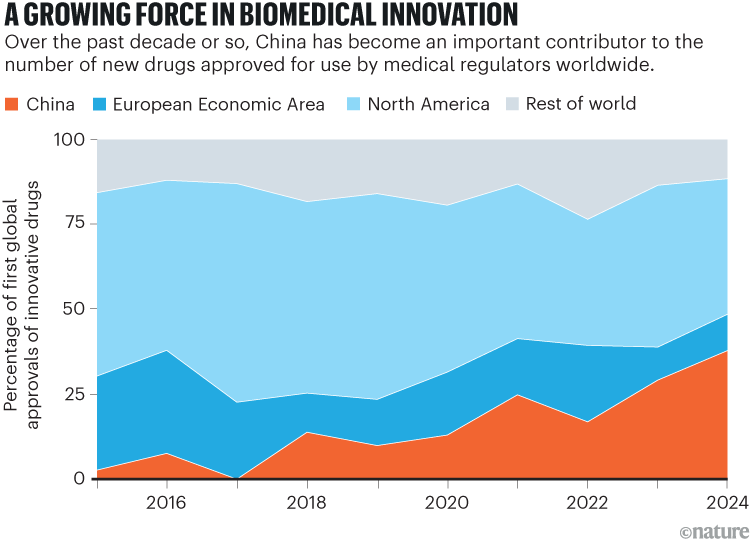
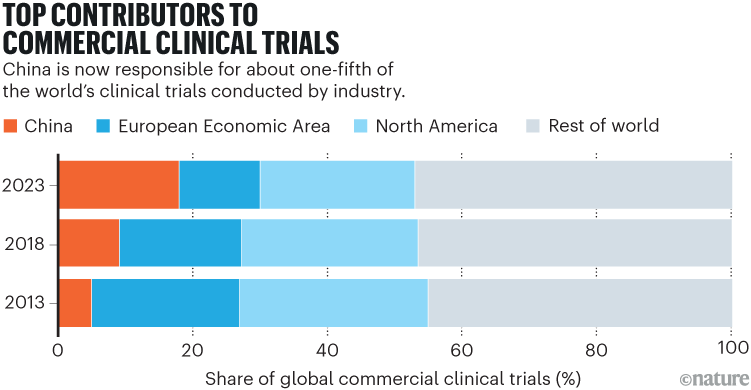
Industry analysts estimate that China now accounts for 70–95% of the global supply chain for many essential pharmaceutical products, including ibuprofen and paracetamol. In 2024, Chinese biotechnology firms developed more than 1,250 new drugs, surpassing the European Union and approaching the US total of roughly 1,440 (see ‘A growing force in biomedical innovation’). In 2018, China conducted only 9% of the clinical trials conducted by companies around the world. Now, it is responsible for about one-fifth of such trials1 (see ‘Top contributors to commercial clinical trials’). And in the past few years, it has achieved several therapeutic milestones.
As China’s biotech industry gathers pace, however, so does geopolitical scrutiny.
Last December, the US Biosecure Act was signed into law in response to concerns about national security. This act prevents US pharmaceutical companies that receive federal funding from working with certain Chinese biotech companies. Such increased outside scrutiny, stemming from ongoing concerns about how genetic and clinical information is handled in China, has been prompting Chinese government officials, state-affiliated think tanks and industry stakeholders to advocate for building a closed ‘secure’ biotech ecosystem in China.
Source: https://doi.org/QQDD
Although a desire to sever ties is understandable, closing off China’s biotech and pharma industries and its preclinical and clinical research from the rest of the world would be scientifically and economically counterproductive. It would blunt China’s momentum, restrict people’s access to life-saving medications in nations around the world and stall innovation globally.
Biomedical progress depends on shared knowledge, diverse patient cohorts and the development of regulations that aligns with global standards. In our view, China — and the rest of the world — should be striving to become more collaborative, not less.
China’s biotech rise
Several domestic shifts have been driving China’s biotech boom.
The country’s advance to become the world’s leading provider of many ‘active pharmaceutical ingredients’ (the biologically active components that produce a medication’s intended therapeutic effect), is thanks to decades of investment in chemical manufacturing, as well as in the transport, storage and export of compounds.
China’s contract-development and manufacturing organizations and genomics-service providers are continuing to underpin global pipelines for drug development — even amid increased US legislative scrutiny. Thanks to China’s manufacturing efficiency, lower regulatory hurdles and ability to recruit large numbers of participants in clinical trials, these organizations can provide services more quickly and cheaply than equivalent ones elsewhere can. (China’s large, centralized hospital networks, for instance, make it easier for researchers to recruit participants and coordinate trials across several sites.) This is the case for preclinical studies; for procedures to assess the physical and chemical characteristics of a drug and ensure its quality; and for assistance with obtaining approval from regulators.
In addition, to better align the country’s biotech and pharma sectors with global standards, the Chinese government launched a slew of regulatory reforms for medical products in 2015. These reforms have helped to make China an attractive option for pharma companies wanting to conduct early-stage clinical trials — particularly for drug development in oncology and immunology, and for trials needing participants from only one country.
Source: Ref. 1
Although it is not without problems, China’s National Reimbursement Drug List is another factor that has helped to keep the costs of the drugs developed and produced in China relatively low. To ensure that medical products are affordable, the Chinese government negotiates notable price cuts with companies that want to get their products listed in China and therefore made available to more people. Listed drugs are covered by state-sponsored insurance schemes, with 2025 updates to the scheme particularly benefiting people with rare diseases and those with chronic illnesses such as diabetes and autoimmune disorders.
Finally, over the past decade or so, there has been a rise in the number of people trained in drug discovery and development in China. Under the China Initiative, implemented by the US government in 2018, thousands of researchers and academics affiliated with China, but working in the United States, faced new restrictions and scrutiny — intended to safeguard US laboratories and businesses from espionage. Anyone receiving funds from China or involved in partnerships with institutions from China, for example, had to declare this to the US government.
The climate of anxiety that this created, combined with recruitment programmes by the Chinese government — which have, since 2008, offered research funds and other benefits to try to entice researchers back to China — seem to have catalysed the return of many US-trained Chinese life scientists2. Many of these people have seeded Chinese biotech start-ups.
So can China go it alone?
Some Chinese government officials and industry stakeholders are arguing that, given all these developments, China could compensate for any tools, materials and knowledge lost as a result of the country cutting biotech and pharma ties with the United States or other countries.
A State Council directive (a high-level administrative order) issued by the Chinese government in September 2025, for example, instructs government procurement offices, which manage the buying of goods and other services for government organizations, to prioritize domestic products. And some policy advisers are already discussing how a ‘closed loop’ of home-grown agencies, such as contract-research organizations, contract-development and manufacturing organizations, regulators and health-insurance companies, could be established in China.
The electronics sector faced a similar challenge in 2022, when US export controls reduced China’s access to materials, such as lithography tools, needed to make cutting-edge semiconductor chips. And China’s response was to pour billions of US dollars into a workaround — chiplet technology. Chiplets are less advanced than conventional chips and easier to manufacture, but can be connected to make a functional system. The Chinese government also supported developers of artificial intelligence in finding ways to work around computing limits, leading to home-grown successes such as the AI start-up Zhipu in Beijing.
How China can become a biotechnology superpower
But ultimately, even China’s advances in semiconductors and AI have depended on importing information, materials, software and manufacturing equipment. And probably more so than in semiconductors and electronics, advances in biology depend on the circulation of ideas, enabled by researchers publishing their work in high-impact journals and attending international conferences.
Although China’s advances in biotech and pharma are impressive, the country is still far from being a self-sufficient biotech superpower.
Several Chinese companies chasing the same leads amid a hypercompetitive corporate culture continues to result in inefficiencies and diminishing returns. Also, the country’s biotech industry continues to drive the incremental optimization of pre-existing treatments rather than groundbreaking discoveries. A 2024 review showed, for example, that nearly 40% of registered clinical trials for cell therapies (which involve transferring cells into a person to treat or prevent disease) conducted in China between 2021 and 2023 focused on known molecular targets3.
China’s biotech filings under the Patent Cooperation Treaty — an international agreement that allows inventors to seek patent protection in multiple countries — surged from 119 in 2010 to more than 1,900 in 2023. By comparison, the EU filed 1,369 and the United States 3,721 applications in 2023. But, in part because of their long history of commanding science, the United States and the EU still lead when it comes to representation in high-impact journals and the discovery of genuinely new mechanisms.

Multinational pharmaceutical firms are investing in drug research and production in China.Credit: AFP via Getty
Besides all these challenges, a lack of international trust continues to be a problem, and tensions are sustained in part by events in China. In 2024, for instance, US intelligence officials reported that Chinese biotech firms had transferred intellectual property of US clients to Chinese authorities without the clients’ consent (see go.nature.com/4twedie).
Lastly, most Chinese biotech and pharma companies lack the capital needed to weather failures in high-risk, early-stage research, or to complete the full innovation cycle from discovery to commercialization, to generating enough returns to sustain innovation.
China’s capital markets are still immature. And although the National Reimbursement Drug List has expanded people’s access to drugs, including in low- and middle-income countries, the list makes it harder for China’s biotech and pharma industries to become profitable. In 2024, the average negotiated discount was roughly 63% — the highest so far.




