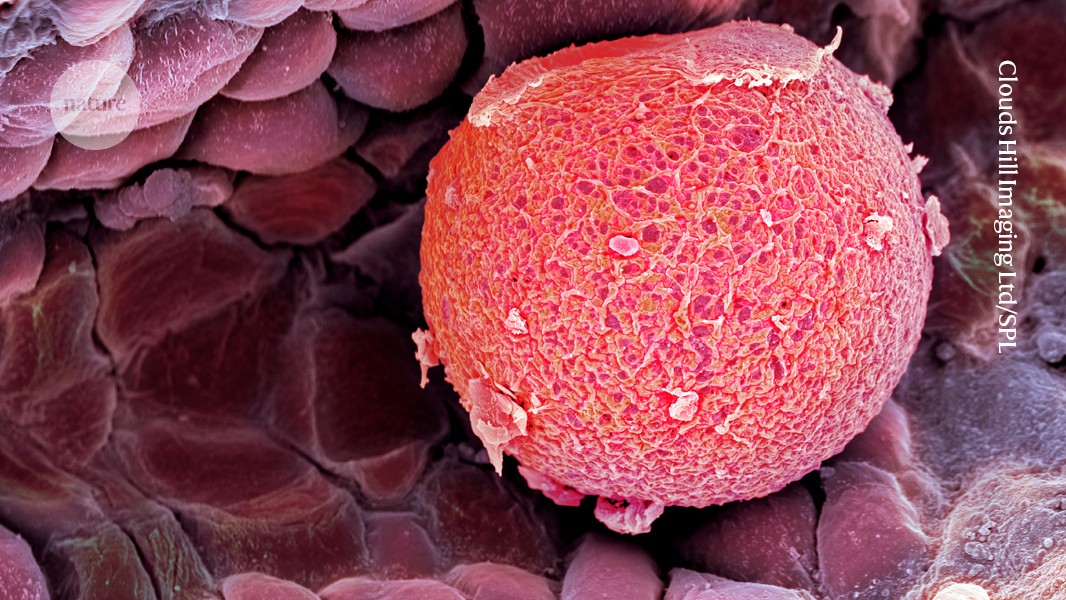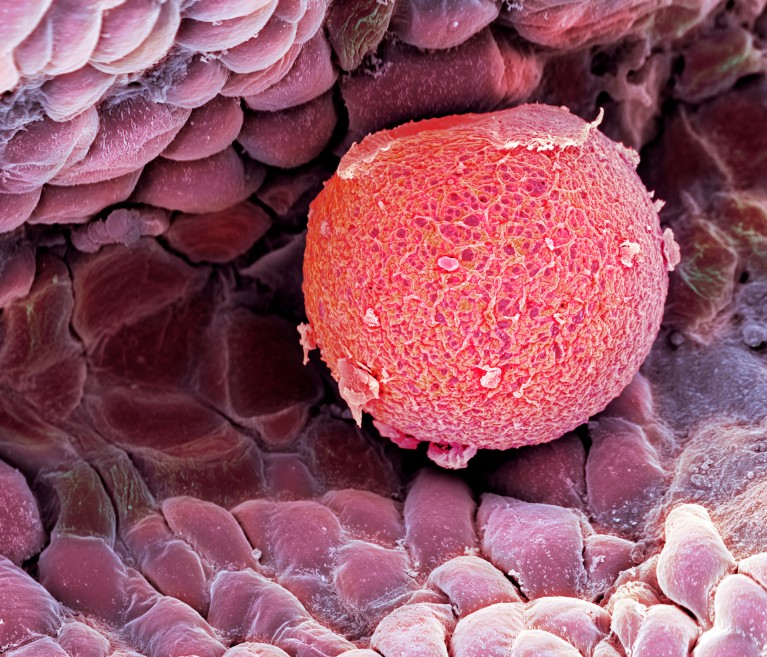
A human fallopian tube cradles an egg (red; artificially coloured). An accumulation of mutations in eggs’ DNA are one possible cause of premature menopause.Credit: Clouds Hill Imaging Ltd/Science Photo Library
Two studies of more than 100,000 women have revealed a suite of genes that help to regulate when a person enters menopause and thus the length of their reproductive span1,2. Some of the genes could also influence the risk of cancer.
Age at menopause can vary widely and is known to be influenced by both environmental and genetic factors. The hope is that these genetic catalogues will help researchers to develop treatments for infertility and create methods for predicting when a person will enter menopause. The studies were published in Nature on 11 September1 and in Nature Genetics on 27 August2.
Rare but powerful
These studies join a bevy of recent efforts to identify genes that contribute to premature menopause. But most of those studies looked for genetic variants that are common in the population, whereas the new projects instead focused on DNA sequences that are rare but which might have a greater effect on ovarian ageing than more-common sequences do.
“They are rare, but typically they are impactful,” says Anne Goriely, a geneticist at the University of Oxford, UK, who was not an author of the papers. “New treatments and conceptual advances often come from these rare disorders.”
Eggs from older mice regain youth when grown in young cells
Hunting for rare genetic variants requires data from a large group of people. To obtain such data, geneticist Anna Murray at the University of Exeter Medical School, UK, a co-author of the Nature paper, and her colleagues relied on the UK Biobank, a massive collection of biomedical data that includes DNA-sequence data as well as information about participants’ lifestyle and health. The researchers focused on protein-coding DNA and found nine genetic variants that are associated with age at menopause. Five of the genes had not been linked to ageing of the ovaries previously.
Women with certain variants in a gene called ZNF518A, for example, were more likely to start menstruating later and undergo menopause earlier than women who did not have those forms of the gene. The result was a reproductive lifespan that was, on average, more than six years shorter.
Mutations for menopause
One factor that could trigger that early menopause is the accumulation of DNA mutations in a person’s eggs. Such mutations can trigger the repair of the eggs’ DNA — or they can cause the eggs to self-destruct. The eggs’ response to DNA damage is key in determining egg number, says Murray. “And it’s egg number that determines your reproductive lifespan.”
Mutations can also increase cancer risk, and variants in four of the genes that the team uncovered were linked not only to early menopause but also to a higher risk of cancer.
Menopause therapy: Brain-based treatment for hot flushes approved by FDA
To look at the relationship between the accumulation of DNA mutations and ovarian ageing, Murray and her colleagues analysed the genetic sequences of more than 8,000 genetic ‘trios’, that of a mother, father and child.
The team found that women who carried common DNA variants that previous research had associated with earlier age at menopause were more likely to pass mutations that had arisen in their eggs to their offspring.
The finding supports the idea that DNA damage is related to ovarian ageing, says Murray. But when the team attempted to repeat their experiment using data from a different biobank, the results were no longer statistically significant.
Even so, the possible links between age at menopause and cancer are important to explore, says Kári Stefánsson, a geneticist and chief executive of the biopharmaceutical company deCODE Genetics in Reykjavik and a co-author on the Nature study. “It focuses the attention on finding a way to deal with conditions like early menopause, and the impact that it has on biology,” he says.
Infertility treatment
In the Nature Genetics study, Stefánsson and his colleagues looked for genetic variants that are linked to early menopause, focusing on variants that had that effect only if they were present in both copies of a woman’s DNA. Their search uncovered a link between age at menopause and a gene called CCDC201, which is known to be active only in immature egg cells2.
Women with certain variants of that gene underwent menopause nine years early on average. The large size of that effect and the specificity of CCDC201 activity suggests that the gene might prove to be a useful target for preventing or treating some cases of infertility, says Goriely. Such an intervention would have to be carefully designed to avoid raising the risk that it would allow eggs to transmit excess damaged DNA to children, but eggs generally contribute many fewer mutations than sperm do anyway, she notes.
“You don’t die of infertility, but for many women who suffer from it, it really is a catastrophe,” Goriely says. “We ought to do something for these women.”
Editor’s note: Nature recognizes that transgender men and non-binary people might undergo menopause. This story uses the term ‘women’ to reflect how participants are reported in the featured studies.