There’s a bar in Baltimore, Maryland, that very few people get to enter. It has a cocktail station, beer taps and shelves stacked with spirits. But only scientists or drug-trial volunteers ever visit, because this bar is actually a research laboratory. Here, in a small room at the US National Institutes of Health (NIH), scientists are harnessing the taproom ambience to study whether blockbuster anti-obesity drugs might also curb alcohol cravings.
Evidence is mounting that they could. Animal studies and analyses of electronic health records suggest that the latest wave of weight-loss drugs — known as glucagon-like peptide 1 (GLP-1) receptor agonists — cut many kinds of craving or addiction, from alcohol to tobacco use.
“We need randomized clinical trials as the next step,” says Lorenzo Leggio, an addiction researcher at the NIH in Baltimore. In the trial he is leading, volunteers sit at the bar and get to see, smell and hold their favourite drinks, while going through tests such as questions about their cravings; separately, participants will have their brains scanned while looking at pictures of alcohol. Some will be given the weight-loss drug semaglutide (marketed as Wegovy) and others will get a placebo.

Addiction researcher Lorenzo Leggio (right) with colleague George Koob in the research laboratory bar at the US National Institutes of Health.Credit: Cliff Owen/AP/Alamy
Curbing addiction isn’t the only potential extra benefit of GLP-1 drugs. Other studies have suggested they can reduce the risk of death, strokes and heart attacks for people with cardiovascular disease1 or chronic kidney ailments2, ease sleep apnoea symptoms3 and even slow the development of Parkinson’s disease4. There are now hundreds of clinical trials testing the drugs for these conditions and others as varied as fatty liver disease, Alzheimer’s disease, cognitive dysfunction and HIV complications (see ‘Diseases that obesity drugs might treat’ at the end of this article).
“We’re in a phase where GLP-1-based drugs are being considered as potential cures for every condition under the Sun,” says Randy Seeley, an obesity specialist at the University of Michigan in Ann Arbor, who has consulted for and received research funding from several firms that develop obesity drugs.
How rival weight-loss drugs fare at treating obesity, diabetes and more
It could take years to prove in which cases the drugs are useful. Understanding how they work could be even harder. In some instances, such as for people with cardiovascular disease, the reason seems straightforward: weight loss is almost certainly providing much of the benefit. But the effects observed in conditions such as addiction and Parkinson’s disease involve other mechanisms that are far from being unravelled.
Working them out, Leggio notes, could help to explain why some people respond better than others to the drugs, and how to mitigate potential side effects, such as nausea, constipation, a reduction in muscle mass during weight loss, and (in rare cases) pancreatitis.
Drugs that work and are safe might be enough for most clinicians and people seeking treatment, says Daniel Drucker, an endocrinologist at the University of Toronto in Canada who consults for and receives research funding from obesity-drug firms. “But if you are trying to capitalize on a possible therapeutic effect and make the next generation of a drug even better, then you ought to know where it is working and how it is working,” he says.
Penetrating the brain
The key property of obesity drugs is that they mimic the natural GLP-1 hormone and activate the same receptors that it would typically target. But because the synthetic drugs are long-lived, their effects extend well beyond those of the hormone they copy5.
There are two natural GLP-1 systems in the body: one in the gut and one in the brain. After each meal, cells in the lining of the intestine produce GLP-1. This stimulates the pancreas to release insulin, which helps to regulate blood-sugar levels, suppress appetite and slow down digestion.
The second system activates only under specific conditions, such as after a large meal or in response to a stressor such as an infection. In these instances, neurons in the hindbrain — the lower back region of the brain, including part of the brainstem — can also produce GLP-1, and there are receptors for the hormone in many neurons across the brain. They include those involved in appetite control, mood regulation, reward and movement.
These systems seem to be entirely separate. It was once thought that gut GLP-1 communicates with the hindbrain neurons by signalling through the vagus nerve (which goes up through the brainstem), but researchers have shown that these systems don’t typically interact6. The gut hormone is quickly metabolized after it is released into the bloodstream: it disappears in only a few minutes.
Synthetic GLP-1 drugs, by contrast, last much longer in the body — a week or more in the case of semaglutide and another drug called tirzepatide. That gives them a better chance of getting into the brain (see ‘Where do obesity drugs act?’).
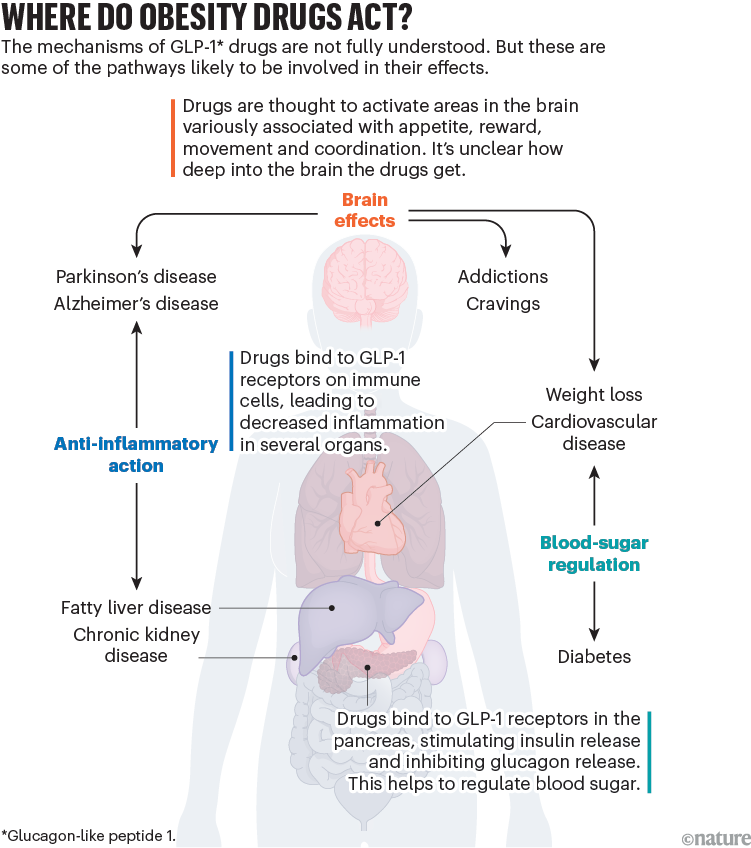
It’s likely that the drugs are targeting GLP-1 receptors both in peripheral organs and in the brain, says Karolina Skibicka, a neuroscientist at Penn State University in University Park, Pennsylvania, and at the University of Gothenburg, Sweden, who has also received funding from and consulted for obesity-drug firms. “That’s a big difference from what our physiology normally does,” she says. “This is part of the reason why these drugs have been so successful in not only treating obesity, but potentially other conditions,” she adds.
It remains unclear how deep into the brain the drugs actually get. Animal studies suggest that some drugs in this class can cross the blood–brain barrier, the protective layer that dictates which substances can move into the brain7. But some scientists say the drugs can’t penetrate deeply and are only able to access certain regions where the blood–brain barrier might be leaky, potentially setting off a cascade of signals from there. (An industry-sponsored study found that semaglutide does not cross the blood–brain barrier8.)
“These drugs are able to activate regions deep within the brain that they clearly can’t penetrate. And this is still a mystery,” says Drucker, whose research on the hormone GLP-1 contributed to the development of the drugs.
Combating cravings
Despite that mystery, it’s becoming clear that obesity drugs might suppress addiction in ways related to how they suppress appetite.
For appetite control, animal studies suggest that the drugs act predominantly on GLP-1 receptors on neurons located in the hypothalamus and the hindbrain. These regions regulate functions such as hunger, body temperature and heart rate.
Obesity drugs aren’t always forever. What happens when you quit?
But that’s not all they do. The drugs also affect neural pathways that govern taste, reward and value — a property that neuroscientists call salience, says Allison Shapiro, a specialist in neurodevelopment at the University of Colorado Anschutz Medical Campus in Aurora. The neurotransmitter dopamine has an important role in these pathways, but it’s not the only player: the circuits are complex and not fully understood.
The drugs’ effects on reward and salience suggest why they might also affect cravings and addiction, says Leggio. They are thought to dampen the brain’s reward system so that an individual might not feel the urge to drink another glass of wine, smoke another cigarette or eat another slice of pizza to get that extra rush of pleasure. This doesn’t necessarily mean that the drugs would reduce people’s ability to feel pleasure, only that they might be less inclined to repeat their behaviours in a continuous search for reward.
There is another factor to consider in the case of addiction, Leggio adds: substances such as alcohol and methamphetamine can disrupt the blood–brain barrier, potentially affecting the action of GLP-1 drugs. “Giving a drug of this kind to someone who is a healthy person may be different from giving the same medication to someone who has an addictive disorder and whose blood–brain barrier has been destroyed,” he says.
GLP-1 medications are being tested as ways to treat multiple substance-use disorders. One small trial — unpublished, but presented at a conference in February — found that people undergoing treatment for opioid-use disorder who took a GLP-1 drug called liraglutide reported a 40% reduction in opioid cravings. Another study is evaluating the potential of exenatide, another GLP-1 mimic, to treat cocaine addiction. Clinical trials are also investigating whether semaglutide, liraglutide and exenatide can help people to stop smoking.

A number of obesity drugs are being trialled for their ability to cut nicotine cravings.Credit: Jakub Porzycki/NurPhoto via Getty
The advantages of weight loss
For some conditions, the benefits of these drugs stem directly from weight loss. People with cardiovascular disease, for instance, are known to benefit from losing weight and consequently shedding accumulated fat that might contribute to clogged arteries. Unsurprisingly, a trial found that people with heart disease who were obese or overweight reduced their risk of having a severe cardiovascular event — including death, stroke or heart attack — by 20% when they took semaglutide1.
Weight loss is also an effective therapy for people with obstructive sleep apnoea, because excessive weight leads to fat deposits in the neck, which can temporarily block airways during sleep. A trial of tirzepatide showed benefits for this condition3.
Cheaper versions of blockbuster obesity drugs are being created in India and China
Perhaps less obviously, weight loss also explains why semaglutide eases a condition that affects the ovaries, called polycystic ovary syndrome (PCOS). Studies had already established that losing weight and following a healthy diet can help to reduce the symptoms of PCOS, which is associated with excess testosterone, irregular or missed periods and insulin resistance, a condition in which the body’s cells have trouble using insulin to absorb sugar (glucose) from the blood.
In a clinical trial presented last year (but not yet published in a journal), researchers treated girls and young women with PCOS who were obese with either semaglutide or an intensive dietician-led programme over four months. Both groups experienced reductions in testosterone and an increased number of periods. In the semaglutide group, the more weight participants lost, the greater their improvements9. “What we found was that semaglutide, as expected, improves glucose metabolism. But all the other reproductive and metabolic improvements were because of the weight loss,” says Melanie Cree, a paediatric endocrinologist at the University of Colorado Anschutz Medical Campus who led the trial.
Taming inflammation
But there are plenty of conditions in which weight loss doesn’t explain the drugs’ benefits. One clinical trial in people with type 2 diabetes and chronic kidney disease found that semaglutide cut the risk of serious kidney complications — including the need for dialysis and transplantation — by 24%. The study2 concluded that the mechanism of kidney protection was unrelated to changes in the participants’ body weight. The authors hypothesize, instead, that the drug acts by reducing inflammation in the kidney.
Semaglutide, marketed as Wegovy, reduced serious kidney complications in a trial in people with type 2 diabetes.Credit: M. Scott Brauer/New York Times/Redux/eyevine
Inflammation occurs when immune cells rush to the site of an injury or illness to start the healing process. But if it becomes chronic, this can contribute to health problems. Animal experiments have demonstrated that drugs that act on GLP-1 receptors can dampen inflammation in the kidneys, heart and liver.
That helps to explain the positive effects of survodutide — a drug that mimics both GLP-1 and glucagon, another hormone involved in blood-sugar regulation — in a type of fatty liver disease called metabolic dysfunction-associated steatohepatitis. In a clinical trial, the drug led to improvements in the condition in 47–62% of participants, depending on the dose they received10.
The mechanism involves the brain as well as the peripheral organs. There are plenty of places in the body where GLP-1 receptors can be found on immune cells, so that’s one obvious route. But in some tissues where the drugs reduce inflammation, there aren’t many GLP-1 receptors around. A study last year found that in such organs, GLP-1 receptors in the brain are likely to be responsible for the anti-inflammatory effects. When researchers blocked GLP-1 receptors in animals’ brains using genetic methods or drugs, the treatments no longer reduced inflammation in multiple tissues, confirming the connection11.
Parkinson’s and Alzheimer’s
The anti-inflammatory effect might also explain how GLP-1 drugs help to ease the symptoms of neurodegenerative diseases such as Parkinson’s4,11 and Alzheimer’s disease. Approved drugs for the conditions don’t target the excessive brain inflammation that is characteristic of these diseases.
In one trial, people with Parkinson’s who took exenatide had significantly improved motor abilities compared to people who received a placebo12. A larger, phase III clinical trial testing the same drug is currently under way, with results expected this year. Tom Foltynie, a neurologist at University College London who is leading the work, started to explore the potential of exenatide in 2008, inspired by basic research demonstrating its neuroprotective properties in animal models.
How anti-obesity drugs cause nausea: finding offers hope for better drugs
Parkinson’s is partially caused by impairments in neurons’ mitochondria, the cellular organelles responsible for energy production. When cells run out of energy, they can’t repair themselves and cell connections stop working. This causes inflammation, which possibly further aggravates the situation, Foltynie says. Some of the affected neurons produce dopamine, which plays a part in movement and coordination and explains the major symptoms of the disease.
Foltynie’s hypothesis is that exenatide reduces inflammation and improves mitochondrial function, which might allow neurons to start working again, improving the motor symptoms.
Christian Hölscher, a neuroscientist at the Henan Academy of Innovations in Medical Science in Zhengzhou, China, says that the clinical evidence on the utility of GLP-1 drugs in Parkinson’s is already compelling and that, if positive, the phase III trial results for exenatide will be a game-changer for clinical practice. Hölscher is the chief scientific officer of Kariya Pharmaceuticals, a Danish biotech firm in Copenhagen that is exploring GLP-1 drugs as a way to treat neurodegenerative diseases.
He is now working on strategies to develop GLP-1 drugs that penetrate the brain in higher concentrations than for currently available drugs. “There’s a clear correlation between the ability to get into the brain and the neuroprotection effect,” he says.
A similar mechanism could explain some promising preliminary results for Alzheimer’s disease, too. Hölscher’s colleagues presented a small, unpublished study at a conference in July suggesting that cognitive decline in people with Alzheimer’s who took liraglutide was 18% slower over a year compared with those who were given a placebo.
Semaglutide is also being evaluated for treating early Alzheimer’s disease in two large clinical trials sponsored by the drug maker Novo Nordisk, based in Bagsvaerd, Denmark.
Every disease?
The list of potential uses for GLP-1 medications doesn’t end there. Because the drugs are thought to act on the neurotransmitter serotonin — the target of many antidepressants — researchers wonder whether they might have the potential to treat depression and anxiety.
For instance, Skibicka and her colleagues studied the effect of both the natural hormone GLP-1 and the drug exenatide in rats, and found that chronic administration of both substances reduced depression-like behaviour in these animals13.
Obesity drugs have another superpower: taming inflammation
It’s not clear whether the effects would be the same for humans, but at least one clinical trial is under way to evaluate semaglutide as a treatment for cognitive dysfunction — difficulties in thinking clearly, concentrating and memory — in people with major depressive disorder.
It’s increasingly hard to find a bodily system that isn’t touched in some way by the drugs. Researchers are also investigating how these drugs might affect fertility and exploring their potential to treat inflammatory conditions such as arthritis.
But the field should remain cautious, says Seeley — particularly when deciding whether people who aren’t overweight or obese should take the drugs, considering their side effects.
And Tamas Horvath, a neuroscientist at Yale University in New Haven, Connecticut, questions the portrayal of these medications as a cure-all — especially because researchers still don’t know what the long-term effects might be. “We don’t know the outcomes of using them continuously for years and decades,” he says.