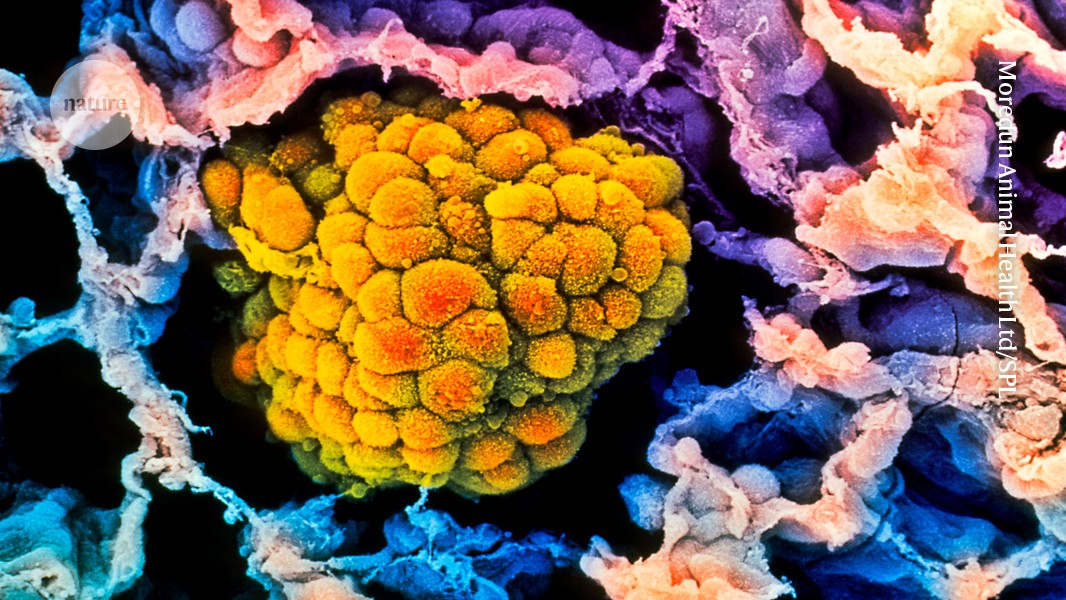
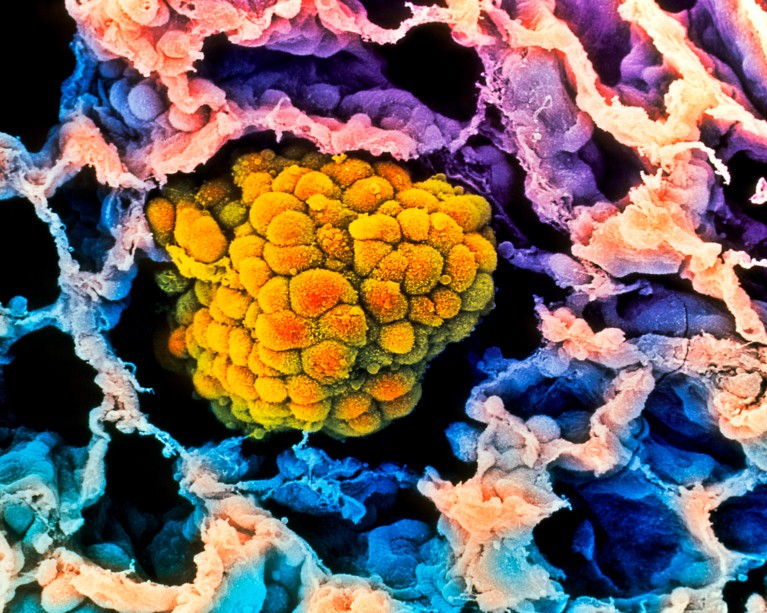
A tumour (artificially coloured) fills the alveolus of a human lung. Some evidence suggests that risk of these cancers decreases with age. Credit: Moredun Animal Health Ltd/Science Photo Library
Becoming an octogenarian could have an unexpected benefit: a decrease in the risk of lung cancer, according to two studies in mice1,2.
The results, posted as preprints on the bioRxiv server, highlight specific genes that could contribute to the declining risk and reveal a surprising link between them and iron metabolism. The studies have not yet been peer reviewed.
The findings might seem counter-intuitive: cancer is a disease associated with ageing, and the likelihood of many cancer diagnoses peaks in a person’s 60s or 70s. But after that, rates of many of those cancers mysteriously decline.
“It’s an observation that we have made for decades,” says Ana Gomes, who studies ageing and cancer at the H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida, and who is not involved in the preprints. “But we have really not been able to explain why that is.”
Age accumulation
Cancer is caused by DNA mutations that accumulate over time. More years of life mean more opportunities to collect the constellation of mutations necessary to generate rogue cancer cells that grow uncontrollably. Immune responses that might once have been able to keep a tumour in check might also become more muted with age.
But the changes to the tissue that come with ageing can also discourage tumour growth by altering the environment in which cancer cells live. Older lungs, for example, tend to have more scar tissue than younger lungs do. Lung cells also become less capable of regeneration, and less resilient to the stresses of unregulated growth. “Structurally and functionally, what you have at an older age is a completely different environment than what you have at young age,” says Gomes.
New lung-cancer drugs extend survival times
To learn more about how ageing affects tumour growth, Emily Shuldiner, a cancer biologist at Stanford University in California, and her colleagues studied mice that have a cancer-causing mutation that the authors controlled with a genetic switch1. The team turned on these mutated genes in the lungs of young and old mice, and found that tumours were larger and more frequent in the younger mice than in the older mice.
The researchers also used CRISPR–Cas9 gene editing in mouse tumours to assess the effects of inactivating each of more than two dozen genes that normally suppress tumour growth. On average, turning off most of these genes increased the rate of tumour growth in mice of all ages, but there were more tumours, and they grew larger, in younger mice than in older mice. This suggests that a different process might be working to suppress cancer in older mice.
Iron grip on tumours
Another team led by Xueqian Zhuang, a cancer biologist at Memorial Sloan Kettering Cancer Center in New York City, found that ageing increases the production of a protein called NUPR1 — which affects iron metabolism — in mouse and human lung cells2. The cells then behaved as if they were iron deficient, limiting their capacity for the rapid growth that is a hallmark of cancer.
To follow up on this finding, the team used CRISPR—Cas9 gene editing to inactivate the Nupr1 gene in older mice. Iron levels in their lungs rose, and the mice became more prone to tumours, like their younger counterparts.
The authors also found that people over the age of 80 have more NUPR1 in their lung tissue than do people under the age of 55, suggesting that the mechanism might be conserved between mice and humans.
The stress of cancer
The results nicely demonstrate that ageing can affect the fitness of lung cancer cells in ways that prevent tumours, says Gomes. But there could be important differences in how tumours are generated in humans and in these mice, she adds. In humans, cancer-causing mutations usually accumulate gradually, and the seeds of a cancer can be planted decades before a tumour is detectable. In the mice, however, tumours were initiated by suddenly switching on the cancer-causing gene when the mice were already old.
And results from lung cancer might not translate to cancers in other tissues, says Cecilia Radkiewicz, an oncologist and cancer epidemiologist at the Karolinska Institute in Stockholm. “It’s quite different between different cancer sites because there have different biological drivers,” she says.
Radkiewicz has found that, in many cancers, the apparent decline in incidence with old age could be an artefact. When she looked at how frequently tumours were found during autopsies, this decline often disappeared3. This suggests that the rates of various cancers often remain the same even during old age, she says, but the cancers are simply diagnosed or reported less often in people over the age of 75.
The pros and cons of screening
An exception, she adds was lung cancer: its incidence did actually decline in older people, even when accounting for autopsy data.
Overall, the findings highlight the importance of studying cancer in aged mice, says Zhuang. Such studies can be difficult, she says: it is expensive and time-consuming to rear mice into old age. But the results could illuminate new ways of treating cancer in old and young people, as well as highlight important targets for regenerative medicine.
“People often think ageing is just bad,” says Dmitri Petrov, an evolutionary biologist at Stanford University and an author on the preprint along with Zhuang. “But if this [work] is correct, then ageing has a beneficial role to play.”