Plasmids and strains
Escherichia coli BL21(DE3) Gold cells (Stratagene) were used for growth analysis, electron tomography and protein expression. For tomography and biochemical experiments, GroEL was expressed from a pBAD33 plasmid containing the groEL gene under the control of an araBAD promotor (EL+ cells)26. For overexpression of GroEL and GroES, a pBAD33 plasmid containing both groEL and groES genes under the control of an araBAD promotor was used48. MetK was expressed from a pET22b plasmid previously described26.
Antibodies
Polyclonal antisera used against GroEL, GroES, MetK and GAPDH were previously described26, and the rabbit antiserum against α-lactalbumin was a product of East Acres Biologicals immunization service.
E. coli growth
E. coli cells were grown in lysogeny broth (LB) medium that contained, depending on the plasmids used, the antibiotics ampicillin (200âμgâmlâ1, pET22b-MetK) and chloramphenicol (32âμgâmlâ1, pBAD33 variants). For overexpression of GroEL, GroES and MetK (MetK cells), transformed E. coli Bl21 (DE3) pBAD33-GroEL:ES pET22b-MetK cells were grown to early exponential phase at 37â°C, and GroELâGroES expression using the pBAD33 promoter was induced for 90âmin by supplementation of LB medium with arabinose to a final concentration of 0.2% (w/v). Cells were subsequently harvested by centrifugation at 8,000g (4â°C for 10âmin) and resuspended to an optical density (600ânm, OD600) of 0.1â0.2 in fresh LB medium containing both antibiotics and 1âmM isopropyl β-d-thiogalactopyranoside (IPTG), to induce MetK expression under control of the T7 promoter for 40âmin. GroEL expression (EL+) was induced in E. coli Bl21 (DE3) pBAD33-GroEL by supplementation of LB medium with arabinose to a final concentration of 0.1% (w/v) and growth of the culture at 37â°C. To expose E. coli Bl21 (DE3) cells to HS, cells were first cultured to early exponential phase at 37â°C and then incubated in a shaking water bath at 46â°C for 2âh.
E. coli growth curves
Cells were cultured as described above. Aliquots were removed at the time points indicated for optical density measurement at OD600. To ensure exponential growth conditions, growing cultures were diluted to an OD600 of 0.1 with prewarmed LB medium containing the necessary antibiotics and arabinose when OD600 just exceeded 0.4. Growth curves for MetK and EL+/MetK cells were measured following termination of GroEL induction by transfer of cells into arabinose-free medium containing 1âmM IPTG for MetK overexpression. The first sample was taken 5âmin after changing the medium. Data were processed for fitting in R.
Protein expression and purification
GroEL, GroES and MetK proteins were expressed and purified as previously described26,49.
Measurement of protein concentration
Concentrations of purified proteins were determined by measurement of absorbance at 280ânm using absorbance coefficients calculated from the protein sequence with the program ProtParam50. Protein concentrations of cell lysates were determined with the Pierce Coomassie Plus (Bradford) Assay Kit (Thermo Fisher Scientific) as described by the manufacturer.
Preparation of cell lysates
Cultures were prepared as described above, harvested by centrifugation and the cell pellet flash-frozen in liquid nitrogen before further processing. Spheroplasts were prepared at 4â°C as previously described51. In brief, cells were resuspended in 100âmM Tris-HCl pHâ8.0 and washed twice with 2âml of buffer. The pellet was then resuspended in HMK buffer (50âmM HEPES-KOH pHâ7.2, 20âmM Mg acetate, 50âmMâK acetate) supplemented with 20% (w/v) sucrose and 0.25âmgâmlâ1 lysozyme. Cells were then incubated on ice for 7âmin and transferred to 37â°C for 10âmin. The resulting suspension was supplemented with Complete EDTA-free protease inhibitor cocktail (Roche), and spheroplasts were lysed by the addition of 0.1% (v/v) Triton X-100 and subsequent sonication.
Mass spectrometry
Cell lysates were reduced by the addition of dithiothreitol (DTT) to a final concentration of 10âmM and heated to 56â°C for 45âmin. Acylation of thiol groups was performed by the addition of chloroacetamide to a final concentration of 55âmM and incubation for 45âmin in the dark, followed by a first digestion step with Lys-C (Wako) at a w/w ratio of 1:20 for 2âh at 37â°C. This was followed by a second digestion step overnight with trypsin (Roche) at a 1:20 (w/w) ratio at 37â°C. The reaction was stopped by the addition of trifluoroacetic acid to a final volume of 1%. Peptides were desalted using OMIX C18 (100âμl) tips (Agilent Technologies, no. A57003100) according to the manufacturerâs instructions.
Desalted peptides were dissolved in 12âµl of 5% formic acid, sonicated in an ultrasonic bath, centrifuged and transferred to autosampler vials (Waters). Samples were analysed on an Easy nLC-1200 nanoHPLC system (Thermo) coupled to a Q-Exactive Orbitrap HF mass spectrometer (Thermo). Peptides were separated on pulled-spray columns (ID 75âμm, length 30âcm, tip opening 8âμm, NewObjective) packed with 1.9âμm C18 particles (Reprosil-Pur C18-AQ, Dr Maisch) using either a stepwise 196âmin gradient (comparison of 37â°C, HS and MetK) or a stepwise 67âmin gradient (all other samples) between bufferâA (0.2% formic acid in water) and bufferâB (0.2% formic acid in 80% acetonitrile). Samples were loaded on the column by the nanoHPLC autosampler at a pressure of 900âbar. The high-performance liquid chromatography flow rate was set to 0.25âμlâminâ1 during analysis. No trap column was used. The following parameters were used for comparison of growth conditions 37â°C, HS and MetK: MS, resolution 60,000 (full-width at half-maximum (FWHM) setting); MS mass range 300â1,650âm/z; MS-AGC-setting 3âÃâ106; MS-MaxIT 50âms; MS/MS fragmentation of the 15âmost intense ions (charge state 2 or higher) from the MS scan; MS/MS resolution 15,000 (FWHM setting); MS/MS-AGC-setting 105; MS/MS-MaxIT 50âms; MS/MS isolation width 1.8âm/z; collision-energy setting 29 (NCE). All other samples were analysed with the following parameters: MS resolution 120,000 (FWHM setting); MS mass range 300â1,650âm/z; MS-AGC-setting 3âÃâ106; MS-MaxIT 100âms; MS/MS fragmentation of the ten most intense ions (charge state 2 or higher) from the MS scan; MS/MS resolution 15,000 (FWHM setting); MS/MS-AGC-setting 105; MS/MS-MaxIT 50âms; MS/MS isolation width 1.2âm/z; collision-energy setting 29 (NCE).
MS data analysis
Protein identification was performed using MaxQuant with default settings. The E. coli K12 strain sequences of UNIPROT (v.2023-03-01) were used as the database for protein identification (Supplementary Information). MaxQuant uses a decoy version of the specified UNIPROT database to adjust false discovery rates for proteins and peptides below 1%.
Quantification of MetK binding to GroEL
To quantify the fraction of GroEL with bound MetK in MetK-overexpressing cells, we immunoprecipitated GroEL with GroEL antibody followed by GroEL and MetK immunoblotting and liquid chromatographyâtandem mass spectrometry. Cells were prepared and lysed as described above, but with the addition of apyrase (25âUâmlâ1 final concentration) to rapidly deplete the ATP pool in the lysate and arrest the GroEL reaction cycle26. The lysate was clarified by centrifugation at 16,000g (4â°C for 10âmin). Either 20âμl of a non-specific antibody (against α-lactalbumin) or a GroEL-specific antibody was coupled to 100âμl of recombinant proteinâA Sepharoseâ4B beads (Thermo Fisher Scientific) as described by the manufacturer. The beads were loaded with sample (180âμg of protein) and incubated in 650âμl of HMK buffer for 1âh. The beads were washed twice with 600âμl of HMK buffer and then twice more with HMK containing 0.1% Triton X-100. For immunoblotting, elution was performed with 50âμl of 2Ãâlithium dodecyl sulfate (Pierce) containing β-mercaptoethanol 5% (v/v) as prescribed by the manufacturer. For liquid chromatographyâtandem mass spectrometry analysis, elution and digestion were performed with the IST MS sample preparation kit (Preomics) using the manufacturerâs on-bead digestion protocol. Mass spectrometry was performed as described above.
SDSâPAGE and immunoblotting
Before SDSâpolyacrylamide gel electrophoresis (SDSâPAGE) analysis, cells were resuspended in HMK buffer supplemented with 2âmM DTT, 1âmM EDTA and 5% glycerol and subsequently sonicated, followed by centrifugation (20âmin, 16,000g at 4â°C). Protein samples were separated by electrophoresis on NuPAGE 10% Bis-Tris SDS gels (Invitrogen) using NuPAGE MES SDS running buffer (Invitrogen) at 150âV. Proteins were transferred to polyvinylidene difluoride membranes in blotting buffer (25âmM Tris, 192âmM glycine, 20% methanol) at 150âmA. Membranes were first incubated with primary antibodies in TBST buffer overnight at 4â°C and subsequently with horseradish peroxidase-conjugated secondary antibody for chemiluminescence detection. Uncropped immunoblots are provided in the Source Data file to Extended Data Fig. 3.
In situ cryo-ET analysis
Cell cultures were grown as described above. For cryo-ET analysis, cells in exponential growth (approximate OD600 0.4) were rapidly (for about 2âmin) concentrated to an approximate OD600 of 10 by centrifugation at 8,000g and subsequently applied to Râ2/1â100 Holey carbon film Cuâ200 mesh grids (Quantifoil) that were previously plasma cleaned for 30âs. The sample was blotted for 9âs at forceâ10 and then plunge-frozen in a mixture of liquid ethane and propane cooled by liquid nitrogen using a Vitrobot MarkâIV (Thermo Fisher Scientific) at 70% humidity and 22â°C. Frozen grids were transferred to a dual-beam, cryo-focused ion beam (FIB)/scanning electron microscope (Thermo Fisher Scientific; either Scios, Quanta, Aquilos or Aquilosâ2). Cells were coated with a layer of inorganic platinum, if available in the system used, followed by the deposition of organometallic platinum using an in situ gas injection system (working distance, 10âmm; heating, 27â°C; time, 8âs). Removal of bulk material was done at a stage angle of 20â25° using gallium ions at 30âkV, 0.5ânA. Fine milling of lamellae was done at 11â13° stage tilt with successively lower currents between 0.3ânA and 30âpA, aiming for a final thickness of 100â200ânm (ref. 52). Lamellae for the selective GroEL overexpression dataset were prepared using Serial FIB53, and an additional layer of inorganic platinum was added following fine milling to avoid charging during image acquisition54. The resulting lamellae were transferred to a TEM (Titan Krios, field emission gun 300âkV, Thermo Fisher Scientific) equipped with an energy filter (Quantum K2, Gatan), a direct detection camera (K2 Summit, Gatan), and tomograms were acquired at a magnification of Ã42,000 (pixel size 3.52âà ), defocus ranging from â5.0 to â3.0âμm and the energy filter slit set to 20âeV using SerialEM 3.9.0 (ref. 55). Tomograms were recorded in dose-fractionated super-resolution mode, with a total dose of roughly 120âeâ/à 2 per tilt series. A dose-symmetric tilt scheme was used with an increment of 2â3° in a total range of ±60° from a starting angle of approximately 10° to compensate for lamellar pretilt (mostly around 11°)56. Frames were aligned using MotionCor2 (v.1.4.0, https://emcore.ucsf.edu/ucsf-software)57. The reconstruction was performed in IMOD using patch tracking (v.4.11.1, RRID:SCR_003297, https://bio3d.colorado.edu/imod/)58 using the TOMOgram MANager (TOMOMAN) wrapper scripts59. Tilt-series images were dose filtered using TomoMANâs implementation of the Grant and Grigorieff exposure filter60. Defocus was estimated using CTFFIND4 (ref. 61).
Tomograms of the EL+ dataset were acquired on a Krios G4 equipped with a SelectrisâX energy filter and Falconâ4 direct electron detector (Thermo Fisher Scientific). Tilt series were collected with a dose-symmetric tilt scheme using TEM Tomographyâ5 software (Thermo Fisher Scientific). A tilt span of ±60° was used with 2° steps, starting at ±10°, to compensate for lamellar pretilt. Target focus was changed for each tilt series in steps of 0.5âµm over a range of â2.5âµm to +5âµm. Data were acquired in EER mode of Falconâ4 with a calibrated physical pixel size of 3.02âà and a total dose of 3eâ/à 2 per tilt over ten frames. A 10âeV slit was used for the entire data collection. Data were preprocessed using TOMOMAN59. EER images were motion corrected using RELIONâs implementation of MotionCor2 (ref. 62). Defocus was estimated using CTFFIND4 (ref. 61). Reconstruction was performed with IMOD using local deposits of the inorganic platinum that was applied by sputtering following milling as fiducials. All tomograms were reconstructed using NovaCTF63.
E. coli membranes were segmented for visualization using TomoSegMemTVâ1.0.
Cryo-ET analysis of in vitro reconstituted GroELâGroES complexes
For generation of a GroELâGroES reference for in situ tomographic analysis containing a defined substrate protein in a folded state and in a known topology, we imaged in vitro reconstituted GroELâGroESâMetK complexes using the same data collection strategy and parameters as above for WT cells.
Subtomogram averaging
For subtomogram averaging, all datasets acquired on the same microscope (37â°C, HS, MetK) were combined and processed together; the EL+ dataset was processed separately. The overall processing workflow is depicted in Extended Data Fig. 1b.
For template matching, PDB entry 1AON was used for ELâES1, 4PKO for ELâES2 and 5MDZ for 70S ribosomes to generate templates at a resolution of 40âà using the molmap64 command in Chimera65. Initial positions for a subset of ELâES1 and ELâES2 complexes and ribosomes were determined using the noise correlation template-matching approach implemented in STOPGAP, by fourfold binning to a pixel size of 14.08âà (ref. 66). This subset of the data was subsequently aligned and classified in STOPGAP to generate a reference from the tomographic data with a Fourier shell correlation (FSC) value close to 1 at 40âà template-matching resolution. Template matching with various GroEL14 species was attempted, but never yielded an average of GroEL14 with a resolution better than the template resolution. The data-derived references of all three different structures were used for an additional round of template matching on the complete dataset. Cross-correlation cut-off was chosen separately for every tomogram by visual inspection of the generated hits and comparison with the tomogram. To reduce the level of false-positive detection, a mask for the cytosol of the cell was first created using AMIRA (Thermo Fisher Scientific) and subsequently used to filter out hits outside of the cytosol. Putative particles were deliberately overpicked with low-resolution templates in the initial stage to avoid false-negative assignments.
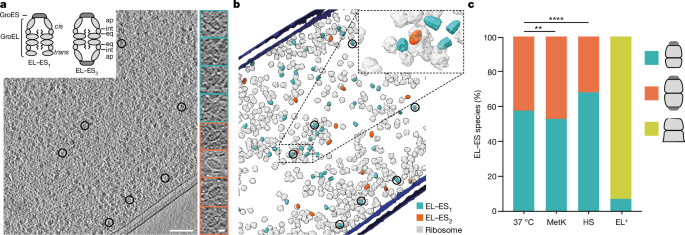
This procedure yielded 176,408âinitial subtomograms for the ELâES1 reference and 125,860 for the ELâES2 reference. These were then further aligned and classified separately in STOPGAP, each yielding classes containing both ELâES1 and ELâES2 particles. The combined number of particles contained in classes with emergent high-resolution features (Supplementary Fig. 1a) for the ELâES1 reference was 19,239, and 17,614 for the ELâES2 reference (Extended Data Fig. 1 and Supplementary Fig. 1b). Because both references pick up a subset of the other particles, the particles were then combined and duplicates removed. The resulting combined dataset was split by reference-free, three-dimensional classification in STOPGAP, resulting in a set of 17,598âELâES1 and 11,213âELâES2 complexes that were then independently refined. This resulted in a resolution at the FSC cut-off of 0.143 following the application of symmetry at 11.6âà for the ELâES1 complex (C7 symmetry) and 11.9âà for the ELâES2 complex (D7 symmetry). Classification was performed using simulated annealing stochastic hill-climbing multireference alignment as previously described67. All classifications were done repeatedly with different, random initial starting sets of 250â500âsubtomograms to generate the initial references. Only particles that ended up in the same class for all independent rounds of classifications were retained67. Further refinements with the established WARP, RELION, Mâpipeline were attempted but did not yield any further improvements. ELâES1 wide and narrow complexes were separated by classification with a focused, disk-shaped mask on the apical domains of the ELâES1 trans-ring. This resulted in 6,681ânarrow complexes that were refined to a resolution of 13.5âà , and 10,130âwide ELâES1 complexes refined to a resolution of 12.0âà .
The EL+ dataset was processed in the same way, but starting with the structures from the other datasets, low-pass filtered to 40âà , as initial references for template matching. Template matching was then repeated once with structures generated by averaging a subset of particles from this dataset. To improve the resolution for model building, the dataset was exported to WARP68 and angles and positions refined using RELION v.3.0.8 (ref. 69). This yielded a GroEL14 structure at a global resolution of 13âà . GroEL 14-mer particles were corefined for geometric distortions with ribosomes in M. The resulting GroEL 14-mer particles were exported for further alignment and classification in RELION. Classification was performed with a regularization parameter T of four and six classes for 25âiterations without angular search, resulting in a more homogeneous subset of 12,421âparticles. These particles were again corefined in M for geometric distortions and per-particle defocus for contrast transfer function (CTF) estimation, resulting in a final structure with nominal resolution of 9.8âà at 0.143âFSC cut-off.
Owing to their high molecular weight and density, ribosome template matching achieves a higher precision and recall. During initial rounds of classification in STOPGAP, because no false-positive particles were detected, all ribosomal hits from template matching were aligned first in STOPGAP at progressively lower binnings (bin4, bin2, bin1). The resulting particles were then exported to WARP using TOMOMAN. Subtomograms were reconstructed for RELION v.3.0.8 using WARP at a pixel size of 3.52âà per pixel. An iterative approach with subtomogram alignment in RELION and tilt-series refinement in M70 were performed until no further improvement in gold-standard FSC was obtained. This resulted in a final structure of the ribosome at a resolution of 8.6âà for the combined 37â°C, HS and MetK datasets, and 6.3âà for the EL+ dataset, which was processed separately.
In vitro cryo-ET data for GroELâGroES complexes were processed analogous to the in situ data, resulting in 39,518âinitial hits for the ELâES2 template and 46,093 for the ELâES1 template, with both sets having a significant overlap. These were then further aligned and classified separately in STOPGAP, yielding 5,832 and 13,688âparticles, respectively, following duplicate removal.
Classification of SP occupancy of GroELâGroES complexes in situ
For the resolution of densities corresponding to substrate proteins in the GroELâGroES chamber we first performed symmetry expansion around the C2 axis of the ELâES2 complexes and aligned the new set of GroELâGroES chambers with the cis-ring of the ELâES1 complexes. The resulting subtomograms of the chambers were then denoised using TOPAZâs three-dimensional pretrained denoising function71. Because initial attempts to classify the interior of the chamber using STOPGAP multireference-based alignment showed only separation by missing wedge, the subtomograms were combined into 5,000ârandom bootstraps containing 250ârandom subtomograms each. These averages were then used to perform k-means clustering with two classes. Bootstraps from the resulting clusters were averaged and used as initial start structures for multireference alignment in STOPGAP. For this, stochastic hill climbing was performed with a temperature factor of 10 for simulated annealing, followed by 40âiterations of multireference alignment with two classes and a mask around the interior of the chamber. This process was repeated five times. Only particles consistently assigned to the same classes were used for a final round of subtomogram averaging, resulting in one class showing weak diffuse density inside the chamber and a second showing strong density near the bottom. Attempts to further subdivide these two classes resulted only in separation based on missing wedge. Because it was not possible to resolve the C7 symmetry mismatch of the substrate and enclosing chamber, final averages were produced for all different biological conditions with C7 symmetry applied to increase the signal-to-noise ratio. The class showing a strong density near the bottom contained 12,255âsubtomograms, the one showing only a weak diffuse density with 24,435 subtomograms for the combined 37â°C, HS and MetK datasets.
In vitro data were processed analogously. The resulting classes were then again split into ELâES1 and ELâES2 complexes corresponding to their substrate state and exported to WARP68. An additional round of alignments was performed in RELION for all different classes and complexes. A prior was set for all angles. Local search was performed with a sigma of 0.5 and search angle of 0.9°. The resulting particles were separately refined in M, correcting for geometrical distortions. Particles were again exported from M70 and signal subtraction preformed in RELION of the trans-ring for ELâES1 and the opposing chambers for ELâES2. Based on their previous classification results in STOPGAP, the refined signal-subtracted, single-chamber complexes were combined in two groups resulting in 7,087âGroELâGroES chambers containing an ordered SP and 14,371 that either contained a disordered SP or were empty. The resulting chambers were again locally refined in RELION using priors and a sigma on all angles, yielding a resolution of 9.4âà for GroELâGroES chambers containing ordered SP and 8.8âà for the remaining chambers.
Cryo-EM single-particle analysis of GroELâGroESâMetK complexes
For generation of substrate-bound GroELâGroES complexes, 4âμM MetK was denatured in the presence of 1âμM GroEL (14-mer) in bufferâA (20âmM MOPS-NaOH pHâ7.4, 200âmM KCl, 10âmM MgCl2, 5âmM DTT) containing 30âmM NaF and 5âmM BeSO4 by first incubation of the mixture at 60â°C for 15âmin and then cooling to 25â°C in a thermomixer (Eppendorf). The addition of 2âμM GroES (7-mer) and 1âmM ATP (pHâ7.0) resulted in stable chaperonin complexes with encapsulated MetK40. Biochemical analysis of this preparation was performed by size exclusion chromatography on a Superdexâ200â3.2/300âGL column. Fractions were analysed by SDSâPAGE electrophoresis (NuPAGE, Bis-Tris 4â12% gels), and MetK loading of GroELâGroES complexes was estimated by mass spectrometry using intensity-based absolute quantification values72. For analysis by mass spectrometry, fractions F1 and F2 (Extended Data Fig. 5a) were analysed separately but intensities pooled for the determination of intensity-based absolute quantification ratios.
GroELâGroESâMetK samples were concentrated tenfold by ultrafiltration using a 100âkDa Amicon centrifugal concentrator (Millipore) at room temperature. As a control, GroEL and GroES were treated identically in the absence of MetK. Before freezing, 1âμl of a n-octyl-β-d-glucopyranoside stock solution (87.5âmgâmlâ1 in bufferâA) was added per 50âμl of sample. For single-particle analysis and in vitro cryo-ET experiments, 4âμl of the sample was applied onto Râ2/1â100 Holey carbon film Cu 200âmesh grids (Quantifoil) previously plasma cleaned for 30âs. This grid was blotted for 3.5âs at forceâ4 and plunge-frozen in a mixture of liquid ethane and propane cooled by liquid nitrogen using a Vitrobot MarkâIV (Thermo Fisher Scientific) at 100% humidity and 4â°C.
Cryo-EM data for the ELâESâMetK dataset were acquired using a FEI Titan Krios transmission electron microscope and SerialEM software55. Video frames were recorded at a nominal magnification of Ã22,500 using a K3 direct electron detector (Gatan), with a total electron dose of around 55âelectrons per à 2 distributed over 30âframes at a calibrated physical pixel size of 1.09âà . Micrographs were recorded within a defocus range of â0.5 to â3.0âμm.
On-the-fly image processing and CTF refinement of cryo-EM micrographs were carried out using the Focus software package73. Only micrographs that met the selection criteria (ice thickness under 1.05, drift 0.4âà â<âxâ<â70âà , refined defocus 0.5âμmâ<âxâ<â5.5âμm, estimated CTF resolution under 6âà ) were retained. Micrograph frames were aligned using MotionCor2 (ref. 57), and the CTF for aligned frames was determined using GCTF74.
The control dataset of GroELâGroES complexes without MetK was acquired similarly but with a nominal magnification of Ã29,000, resulting in a calibrated pixel size of 0.84âà .
Image processing, classification and refinement for single-particle analysis
From the resulting 8,945âmicrographs of the GroELâGroESâMetK dataset, 1,561,482âparticles were picked using a trained crYOLO network75 and extracted with RELION v.3.1.3 (ref. 69). An initial round of two-dimensional classification was performed and the remaining particles were passed into CryoSPARC76 for further two-dimensional classification, ab initio model building, alignment and initial three-dimensional classification to separate ELâES1 from ELâES2 complexes. The remaining ELâES2 (659,866âparticles) and ELâES1 (294,250âparticles) complexes were then exported separately to RELION for additional alignment with imposed symmetry, CTF refinement and Bayesian polishing. For the ELâES2 complexes, symmetry expansion around the C2 axis was performed and the opposing half removed using RELIONâs signal subtraction.
The resulting asymmetric ELâES1 complexes were then classified further with CryoDRGN77, resulting in a clean subset of 242,276âparticles. The trans-rings of the ELâES1 complexes were classified in CryoSPARC using a focused mask on the apical domains of the trans-ring, resulting in 169,454âparticles in the narrow conformation and 34,755 in the wide conformation. The resulting structures were refined in CryoSPARC under the application of C7 symmetry to a nominal resolution of 2.9 and 3.1âà , respectively. For the analysis of the cis-chamber, all ELâES1 particles were pooled and the trans-ring was removed by signal subtraction in RELION.
The resulting GroES-bound, single-ring particles (1,562,002âparticles) were then aligned to a common reference in RELION and exported to CryoSPARC for further alignment without imposed symmetry. The resulting mask and reference were reimported into RELION and used for an additional alignment step with the goal of aligning the asymmetric MetK substrate contained inside the chamber (Extended Data Fig. 5d). Subsequently a second round of signal subtraction was performed and the resulting particles, comprising only MetK density, were further subjected to three-dimensional classification without angular search in RELION. A subset of the resulting classes showed visible secondary structure elements in different orientations (Extended Data Fig. 5d). These classes were then combined and aligned into a single frame of reference in Matlab 2015b by manual rotation with the respective multiple of 360°/7 around the sevenfold symmetry axis. This was done by adding the corresponding increment to particle rotation angles in the particle table (.star file).
These folded MetK (fMetK) particles were then further locally aligned in CryoSPARC. An additional round of three-dimensional classification was performed followed by a final round of local alignment (322,800âparticles), resulting in density for MetK at a resolution of 3.7âà .
For the study of MetK contacts with the inner wall of GroELâGroES chamber, we reverted the signal subtraction in RELION to generate single-ring GroELâGroESâMetK particles for both the folded MetK and mixed population of chambers either containing disordered MetK or empty; both were refined and aligned in CryoSPARC. The subset containing a mixed population was additionally classified in CryoDRGN between the final alignment steps, resulting in a global resolution of 3.04âà for the GroELâGroESâMetK complex containing folded MetK and of 2.94âà for the complex containing a mixed population of disordered MetK or empty chambers.
GroELâGroES complexes without MetK were processed analogously but without Bayesian polishing and CTF refinement in RELION. Signal subtraction was performed in CryoSPARC; using 293,974âparticles, this resulted in a map with a global resolution of 2.5âà following the application of C7 symmetry.
Densities were visualized and rendered using ChimeraX78,79.
Model building and refinement
Model building was initiated by rigid-body fitting the GroEL subdomains, GroES and MetK from the crystal structures PDB 1SX3 (ref. 80), 5OPW12 and 7LOO42, respectively, into cryo-EM density, followed by manual editing using Coot81. The models were subsequently refined in real space with Phenix82. For the refinement of models against low-resolution data from STA, automatically generated restraints from reference structures such as PDB 8P4M (this study) were used. Residues with disordered sidechains were truncated at C-beta.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.