Mice
C57BL/6 (000664), OT-I (003831), SMARTA (030450), Rosa26-Cas9 knock-in (026179), Cd8Cre (008766) and Rag1−/− (002216) mice (all on the C57BL/6 background) were purchased from the Jackson Laboratory. OT-I mice were crossed with Rosa26-Cas9 knock-in mice to generate OT-I-Cas9 mice. To generate Ifngfl/fl mice, loxP sites were inserted into intron 1 and the 3′ UTR of the Ifng gene, resulting in Cre-mediated deletion of exons 2–4, which were then bred with Cd8cre mice to generate Cd8CreIfngfl/fl mice. Sex- and age-matched (6–10-week-old) mice with predetermined genotypes (not blinded to investigators) were randomly assigned to control and experimental groups throughout the study, and both male and female mice were used. To generate complete bone marrow chimeras, bone marrow cells from Cd8CreIfngfl/fl or control Ifngfl/fl mice were flushed from mouse tibias and femurs, and red blood cells were lysed using ACK lysis buffer, followed by intravenous injection into sublethally (5.5 Gy) irradiated Rag1−/− recipient mice. Mice were inoculated with the indicated tumours at 8 weeks after bone marrow reconstitution. All of the mice were maintained under specific-pathogen-free conditions in the Animal Resource Center at St. Jude Children’s Research Hospital. The animals were housed under 12 h–12 h light–dark cycles coinciding with daylight in Memphis, TN, USA (light on at 06:00 and off at 18:00). Food and water were provided ad libitum. The St. Jude Children’s Research Hospital Animal Resource Center was maintained at 20–25 °C and 30–70% humidity. The research conducted in this study complied with all of the relevant ethical regulations. Experiments and procedures were approved by and performed in accordance with the Institutional Animal Care and Use Committee (IACUC) of St. Jude Children’s Research Hospital. The number of mice per group were selected based on previous publications29,49,61.
Cell lines
B16-OVA, MC38-OVA and MC38 cell lines were provided by D. Vignali. The HEK293T and LoVo cell lines were purchased from the American Type Culture Collection (ATCC). The Plat-E cell line was provided by Y.-C. Liu. All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) or RPMI-1640 medium (Gibco) supplemented with 10% (v/v) FBS and 1% (v/v) penicillin–streptomycin at 37 °C with 5% CO2. No commonly misidentified cell lines (International Cell Line Authentication Committee) were used in this study. Cell lines were tested and determined to be free of mycoplasma contamination. The aforementioned cell lines were not independently authenticated.
Plasmid and stable cell line generation
The sgRNAs targeting Vdac2, Casp3, Casp7, Gsdme, Ifng, Tnf, Tnfrsf1a, Prf1, Ifngr1, Ifngr2, Jak1, Ptpn2, Vdac1, Vdac3, Cgas, Sting1, Mavs, Irf3, Ccl5, Bak1, Bax, Apaf1 and Casp9 or non-targeting control were synthesized, annealed and ligated into BbsI-HF-digested (R3539L, NEB) retroviral sgRNA vectors (LMA or pSIR-DsRed (BbsI), with Ametrine or DsRed as a selection marker, respectively)18,62. A list of the sgRNA sequences is provided in Supplementary Table 10. For the mouse liver tumour model, pX330-sgNTC and pX330-sgVdac2 plasmids were generated from pX330 (42230, Addgene) according to the established protocol63. To generate Cas9-expressing tumour cell lines (B16-OVA-Cas9, MC38-OVA-Cas9 and MC38-Cas9), lentivirus was produced by co-transfecting Lenti-Cas9-GFP (86145, Addgene) plasmid with psPAX2 (12260, Addgene) and pMD2.G (12259, Addgene) packing plasmids into HEK293T cells. The supernatant containing viral particles was collected at 48 h after transfection. B16-OVA, MC38-OVA and MC38 cells were transduced with viral supernatant for 48 h in RPMI-1640 (for B16-OVA) or DMEM (for MC38-OVA and MC38) + 10% (v/v) FBS supplemented with 10 μg ml−1 polybrene (Sigma-Aldrich), followed by sorting of transduced (GFP+) into single clones, followed by expansion. Cas9 expression was verified by immunoblot analysis61. To generate tumour cells deficient for the indicated genes using CRISPR–Cas9, retrovirus was produced by co-transfecting the indicated LMA or pSIR-DsRed (BbsI) vector(s) with pCL-Eco (12371, Addgene) and VSV.G (14888, Addgene) packing plasmids into Plat-E cells. The supernatant containing viral particles was collected 48 h after transfection. B16-OVA-Cas9, MC38-OVA-Cas9 or MC38-Cas9 cells were transduced with viral supernatant for 48 h in RPMI 1640 (for B16-OVA) or DMEM (for MC38-OVA and MC38) + 10% (v/v) FBS supplemented with 10 μg ml−1 polybrene (Sigma-Aldrich), followed by sorting Ametrine+ or Ametrine+DsRed+ (for dual targeting) cells. Cells were cultured for another 14 days for genome editing and expansion. Unless otherwise noted, Cas9-expressing tumour cells were used for all of the experiments described in this study.
To generate the pMIG-II-HA-VDAC2 plasmid used for VDAC2–BAK interaction analysis in B16-OVA cells that did not express Cas9 (see the ‘Immunoprecipitation and immunoblot analysis’ section below), the Vdac2 coding sequence was PCR-amplified from B16-OVA cDNA and cloned into the pcDNA3.1-HA vector (128034, Addgene). The HA-Vdac2 coding sequence was amplified and cloned into the pMIG-II (52107, Addgene) retroviral vector. To generate the pMIA-Flag-BAK and pMIA-Flag-BAX plasmids, Flag-Bak1 and Flag-Bax coding sequences were PCR-amplified from pcDNA-Flag-BAK or pMIG-BAX (8788, Addgene) and cloned into pMIA (52113, Addgene). To generate wide-type HA-VDAC2 plasmid, the HA-Vdac2 sequence with 6 amino acid synonymous mutation at sgVdac2 targeting sequence (to circumvent CRISPR–Cas9-mediated cleavage; ATCCATGGGTCAGCTGTCTTTGGT changed (bold bases) to ATACACGGATCGGCAGTATTTGGT) was first synthesized by Integrated DNA Technologies (IDT). On the basis of this CRISPR–Cas9-resistant VDAC2 construct, we designed three VDAC2 mutants to alter specific sequences (A172R, T168N and D170E (T168N/D170E) or T168N, D170E and A172R (T168N/D170E/A172R) reported to have reduced ability to bind BAK40,41, and these were synthesized by IDT. The following sequences were used for introducing such mutations: (1) A172R (GCC > CGC); (2) T168N/D170E (ACCTTTGAC > AACTTTGAA); (3) T168N/D170E/A172R (ACCTTTGACAGTGCC > AACTTTGAAAGTCGC). All of the plasmids were cloned using the NEBuilder HiFi DNA Assembly Cloning Kit (E5520S, NEB). To generate stable B16-OVA cells with Omi-mCherry (for imaging analysis), HA-VDAC2 and/or Flag-BAK/BAX overexpression (for immunoprecipitation), retrovirus was produced by co-transfecting pBabe(puro)-Omi-mCherry (48685, Addgene), pMIG-II-HA-VDAC2 or pMIA-Flag-BAK/BAX plasmid with pCL-Eco (12371, Addgene) and VSV.G (14888, Addgene) packing plasmids into plat-E cells. Omi-mCherry-, HA-VDAC2-, Flag-BAK- or Flag-BAX-expressing cells were sorted based on the fluorescence reporter mCherry (for Omi-mCherry), GFP (for HA-VDAC2) or Ametrine (for Flag-BAK or Flag-BAX).
T cell-mediated tumour cell killing assay in vitro
In total, 1 × 105 tumour cells were seeded into a 12-well plate for the timepoints indicated in the figures and their legends. Naive OT-I CD8+ T cells were isolated from the spleen and peripheral lymph nodes of OT-I mice and activated using 10 μg ml−1 anti-CD3 (2C11, Bio X Cell, BE0001-1) and 5 μg ml−1 anti-CD28 (37.51, Bio X Cell, BE0015-1) antibodies as previously described18. Activated OT-I cells were then expanded in Click’s medium (Irvine Scientific) containing 10% dialysed FBS supplemented with glutamine in the presence of human recombinant IL-2 (20 IU ml−1; PeproTech), mouse IL-7 (12.5 ng ml−1; PeproTech) and IL-15 (25 ng ml−1; PeproTech) for 2–3 days. Preactivated OT-I CD8+ T cells were then cocultured with tumour cells at the indicated effector:tumour target ratios. The live tumour cell number was calculated, and the mean value of E:T = 0:1 group was set equal to 100%. Fresh human leukapheresis products were purchased from Charles River. These leukapheresis products were obtained from three de-identified healthy donors (donor numbers ECT026, ECT028 and ECT031) and were used to generate human CAR T cells (ECT24-PD030) by St. Jude Experimental Cellular Therapeutics Laboratory (ECTL), using an established protocol and a previously described lentiviral vector that encodes a B7-H3-CAR with a CD28ζ signalling domain64. Generated CAR T cells were cryopreserved at the end of production. As de-identified leukapheresis products were used, CAR T cell generation and experiments with these cells are considered non-human subject research. This determination was confirmed by the Institutional Review Board (IRB) at St. Jude Children’s Research Hospital. Before conducting cytotoxicity assays, CAR T cells were thawed and cultured in X-VIVO-15 medium (BEBP04-744Q, Lonza) containing 5% human serum (H4522, Sigma-Aldrich) in the presence of human recombinant IL-7 (10 ng ml−1, 130-093-764, Miltenyi) and human recombinant IL-15 (10 ng ml−1, 130-095-362, Miltenyi) for 24 h. Recovered CAR T cells were then co-cultured with sgNTC- or sgVDAC2-transduced LoVo cells65 at the indicated B7-H3-CAR T effector:tumour target ratios. At the indicated timepoints of co-culture, the number of live tumour cells were counted by flow cytometry using CountBright Absolute Counting Beads (C36950, Invitrogen).
T cell purification, differentiation and viral transduction for in vitro assays and adoptive transfer into tumour-bearing mice
Naive Cas9-expressing OT-I CD8+ T cells were isolated as mentioned above. Purified naive OT-I cells were activated in vitro for 18–20 h with 10 μg ml−1 anti-CD3 (2C11, Bio-X-Cell), 5 μg ml−1 anti-CD28 (37.51; Bio-X-Cell) before viral transduction. Viral transduction was performed by spin-infection at 900g at 25 °C for 3 h with 10 mg ml−1 polybrene (Sigma-Aldrich). After transduction, cells were cultured in T cell medium containing human recombinant IL-2 (20 IU ml−1; PeproTech), mouse recombinant IL-7 (12.5 ng ml−1; PeproTech) and mouse recombinant IL-15 (25 ng ml−1; PeproTech) for 4 days. Naive Cas9-expressing CD4+ T cells were isolated from the spleen and peripheral lymph nodes of Cas9-SMARTA mice as previously described66. Viral transduction was performed by spin-infection at 900g at 25 °C for 3 h with 10 mg ml−1 polybrene (Sigma-Aldrich). After transduction, cells were cultured for iTreg or TH1 differentiation: naive CD4+ T cells were stimulated with 5 μg ml−1 anti-CD3 (2C11; Bio-X-Cell), 5 μg ml−1 anti-CD28 (37.51; Bio-X-Cell) in the presence of human IL-2 (100 U ml−1) plus human TGFβ (0.5 ng ml−1; PeproTech) for iTreg polarization; or human recombinant IL-2 (100 U ml−1) plus mouse recombinant IL-12 p40 (0.5 ng ml−1; BD Biosciences) for TH1 polarization for 5.5 days. Transduced cells were sort-purified based on the expression of Ametrine.
For assays involving IFNγ or anti-IFNγ treatments of sgNTC- or sgVdac2-transduced OT-I, iTreg or TH1 cells, Ametrine+ sorted cells were incubated with IFNγ (10 ng ml−1) or anti-IFNγ (10 μg ml−1) for 12–24 h as indicated in the figure legends. Cells were collected for flow cytometry, immunoblotting or quantitative PCR with reverse transcription (RT–qPCR) analysis as indicated in the figure legends. For adoptive transfer of sgNTC- or sgVdac2-transduced OT-I cells into B16-OVA tumour-bearing mice, C57BL/6 mice were s.c. injected with 3 × 105 B16-OVA melanoma cells on day 0. At day 12 after tumour inoculation, a total of 4 × 106 sgNTC-transduced (labelled with Ametrine) and sgVdac2-transduced (labelled with Ametrine) OT-I cells were injected intravenously into separate B16-OVA tumour-bearing mice. Tumour growth and mouse survival were monitored.
Cas9+ MEF isolation, transduction and treatment
Cas9-expressing mouse embryos were isolated from Cas9-transgenic mice on E14.5. The embryos were euthanized by decapitation, and the fetal liver and heart were removed with forceps, followed by rinsing of the embryos with ice-cold PBS. The embryos were incubated with 3–5 ml ice-cold Trypsin-EDTA (25200-56, Gibco) overnight on ice in a 50 ml conical tube (Falcon). The trypsin-EDTA was aspirated off the embryos, followed by resuspension in 2 ml of pre-warmed (37 °C) trypsin-EDTA and incubation for 5–7 min in a 37 °C water bath. The digestion reaction was stopped by addition of 10 ml of MEF medium (DMEM + 10% FBS) followed by pipetting without introduction of air bubbles. After resting for 5 min at room temperature, the embryo suspension was transferred to a new 50 ml conical tube and centrifuged at 1,500 rpm for 5 min. The cell pellet was resuspended in MEF medium and filtered through a 70 μm cell strainer to remove debris. The cells from each embryo were plated into one T-160 plates or three T-75 (or 10 cm plates), reaching around 70% confluency within approximately 2 days. MEFs were transduced with sgNTC- or sgVdac2-expressing retrovirus for 48 h in MEF medium containing 10 μg ml−1 polybrene (Sigma-Aldrich), followed by sorting Ametrine+ MEFs. Ametrine+ transduced MEFs were cultured for another 14 days for genome editing and expansion. For assays involving IFNγ (10 ng ml−1, 24 h), ΤNF (10 ng ml−1) plus cycloheximide (5 μg ml−1, 4 h), or etoposide (20 μM, 24 h) treatments, sgNTC- or sgVdac2-transduced MEFs were treated for the indicated times listed above, followed by flow cytometry, immunoblotting or RT–qPCR analysis, as indicated in the figure legends.
Tumour models and immunotherapeutic treatments
Mice (C57BL/6 mice, Cas9+ transgenic mice, Rag1−/− mice or complete bone marrow chimeras) were injected s.c. with 1 × 106 B16-OVA-Cas9, MC38-OVA-Cas9 or MC38-Cas9 cells expressing the indicated sgRNAs in the right flank. For the lung metastasis model, 1 × 106 B16-OVA-Cas9 cells transduced with the indicated sgRNAs were resuspended in 100 μl phosphate-buffered saline (PBS, Gibco) and injected into Cas9+ transgenic mice through the tail vein. After tumour inoculation, mice were randomly assigned to different groups for ICB and/or ACT treatments. For tumour models with OT-I T cell transfer, preactivated OT-I cells (the details are provided above) were transferred intravenously into tumour-bearing mice at day 7 after tumour inoculation (1 × 107 OT-I cells per mouse). Anti-PD-L1 antibody (10 F.9G2, Bio X Cell) or IgG isotype control (LTF-2, Bio X Cell) was injected intraperitoneally three times at a dose of 100 μg in 100 μl PBS on days 7, 10 and 13 after inoculation of B16-OVA-Cas9 cells transduced with the indicated sgRNAs, as described previously61. Anti-PD-1 antibody (J43, Bio X Cell) or rat IgG isotype control (LTF-2, Bio X Cell) was injected intraperitoneally three times at a dose of 100 μg in 100 μl PBS on days 7, 9 and 11 after inoculation of MC38-Cas9 cells expressing the indicated sgRNAs, as described previously61. Mice that completely rejected tumours were rechallenged with 1 × 106 B16-OVA-Cas9-sgNTC or MC38-Cas9-sgNTC cells on day 40 or day 50. Anti-CD8α antibody (2.43, Bio X Cell) or rat IgG isotype control (LTF-2, Bio X Cell) was injected intraperitoneally at a dose of 200 μg in 100 μl PBS on days −1, 2, 5, 8 and 11. Anti-IFNγ antibody (XMG1.2, Bio X Cell) or IgG isotype control (HRPN, Bio X Cell) was injected intraperitoneally at a dose of 200 μg in 100 μl PBS on days −1, 3, 7, 11 and 15 or the timepoints as indicated in the figures and their legends. Emricasan was dissolved in PBS and tumour-bearing mice were treated with emricasan (or PBS vehicle) intraperitoneally at 20 mg kg−1, twice a day for 3 days43. To establish the constitutively active AKT and NRAS-driven liver tumour mouse model, 6-week-old male mice were injected with 5 μg pT3-EF1a-myrAKT1-HA (31789, Addgene), 5 μg pT-Caggs-NRASG12V (20205, Addgene) and 2.5 μg pCMV(CAT)T7-SB100 (34879, Addgene) as previously described67. To target Vdac2 in vivo, 50 μg pX330-sgNTC or pX330-sgVdac2 was mixed together with the above oncogenic vectors and injected into mice. A volume of plasmid solution equal to 10% of the body weight in sterile Ringer’s solution was injected through the tail vein within 5–7 s67. s.c. B16-OVA, MC38-OVA and MC38 tumours were measured every 2 days with digital callipers and the tumour volumes were calculated using the formula: length × width × width × π/6. To isolate intratumoural lymphocytes, s.c. tumours were collected on the indicated days after inoculation, excised, minced and digested with 1 mg ml−1 collagenase IV (LS004188, Worthington Biochemicals) and 200 U ml−1 DNase I (DN25-1G, Sigma-Aldrich) for 1 h at 37 °C and passed through 70-μm filters to remove undigested tumour tissues. TILs from MC38-OVA tumours were further isolated by density-gradient centrifugation over Percoll (17089101, Cytiva). Tumour size limits were approved to reach a maximum of 3,000 mm3 or ≤20% of body weight (whichever was lower) by the IACUC of St. Jude Children’s Research Hospital.
CRISPR–Cas9 mutagenesis screening using the lentiviral metabolic library
Lentiviral sgRNA metabolic library construction
The mouse metabolic library containing 3,017 genes was synthesized based on the gene list from reported human metabolic-associated genes, and library synthesis, purification and quality control were described previously18. In brief, 6 sgRNAs were designed for each gene and were split into two sub-libraries (AAAQ05 and AAAQ07), with each containing 3 sgRNAs targeting one gene and 500 non-targeting controls.
In vitro and in vivo screens
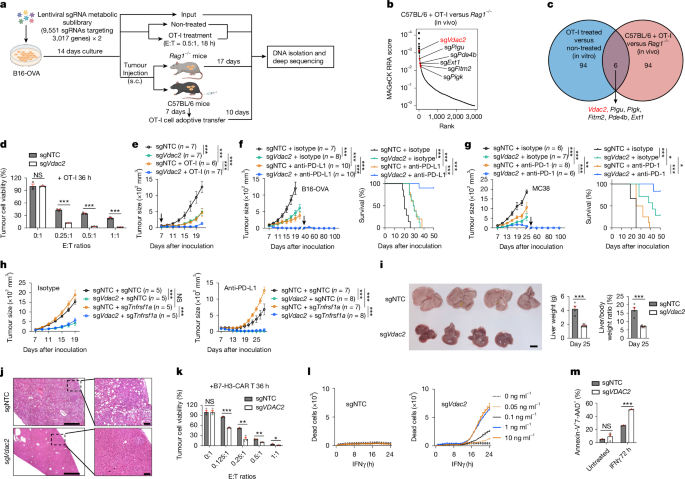
Lentivirus was produced by co-transfecting HEK293T cells with the two lentiviral metabolic sublibrary plasmids, psPAX2 (12260, Addgene) and pCAG4-Eco (35617, Addgene). Then, 48 h after transfection, the supernatant containing viral particles was collected and frozen at −80 °C. A single clone of B16-OVA-Cas9 cells with high Cas9-editing activity was expanded and transduced with the two sub-pools at a multiplicity of infection (MOI) of 0.2–0.3 to achieve 20–30% transduction efficiency. The sublibrary-transduced B16-OVA-Cas9 cells were purified by sorting of Ametrine+ cells and then mixed at 1:1 ratio. Cells were cultured in vitro for another 14 days for genome editing and expansion. An aliquot of 5 × 106 transduced B16-OVA-Cas9 cells (about 250× cell coverage per sgRNA) was saved as the input. For in vitro screening, transduced B16-OVA-Cas9 cells were co-cultured with preactivated OT-I CD8+ T cells (see details above) for 18 h. The remaining tumour cells (5 × 106, about 250× cell coverage per sgRNA) were sorted and used for deep sequencing analysis. For in vivo screening, 1 × 106 transduced B16-OVA cells were inoculated into Rag1−/− mice or Cas9+ transgenic mice (10 mice each group, 2 replicates). Preactivated OT-I cells (the details are provided above) were transferred intravenously into B16-OVA tumour-bearing mice at day 7 after tumour inoculation (1 × 107 OT-I cells per mouse). At day 17 after tumour challenge, Ametrine+ tumour cells were collected from the pooled tumour tissues using a cell sorter. At least 5 × 106 sorted B16-OVA cells (>250× cell coverage per sgRNA) were used for deep sequencing analysis.
Sequencing library preparation
Genomic DNA was extracted by using the DNeasy Blood & Tissue Kits (69506, Qiagen). Primary PCR was performed using the KOD Hot Start DNA Polymerase (71086, Millipore) and the following pair of Nextera next-generation sequencing (NGS) primers: (Nextera NGS forward (-F): TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGTTGTGGAAAGGACGAAACACCG; Nextera NGS reverse (-R): GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGCCACTTTTTCAAGTTGATAACGG). Primary PCR products were purified using the AMPure XP beads (A63881, Beckman). A second PCR reaction was performed to attach Illumina adaptors and indexes to barcode each sample. Hi-seq 50-bp single-end sequencing (Illumina) was performed for library sequencing.
Secondary genome-scale CRISPR–Cas9 mutagenesis screening in VDAC2-deficient tumour cells
In vitro screening after IFNγ treatment
Lentivirus was produced by co-transfecting HEK293T cells with lentiviral genome-scale Brie library plasmids with the puromycin-resistance gene68, psPAX2 (12260, Addgene) and pCAG4-Eco (35617, Addgene). Then, 48 h after transfection, the supernatant containing viral particles was collected and frozen at −80 °C. VDAC2-deficient B16-OVA-Cas9 cells (transduction efficiency, ~5%) were subsequently transduced with the Brie library at an MOI of 0.2–0.3. Brie-library-transduced VDAC2-deficient B16-OVA-Cas9 cells were then cultured with 4 μg ml−1 puromycin for another 14 days to select for transduced cells. An aliquot of 8 × 106 transduced VDAC2-deficient B16-OVA-Cas9 cells (about 100× cell coverage per sgRNA) were saved as input. In total, 5 × 107 transduced VDAC2-deficient B16-OVA-Cas9 cells were treated with IFNγ (554587, BD) at 10 ng ml−1 for 24 h, resulting in more than 50% tumour cell death (2 replicates). Transduced VDAC2-deficient B16-OVA-Cas9 cells without IFNγ treatment were used as control. A total of 8 × 106 transduced VDAC2-deficient tumour cells (about 100× cell coverage per sgRNA) was collected and used for deep sequencing. DNA exaction and sequencing library preparation were as described in the ‘Sequencing library preparation’ section using Q5 enzyme (M0541L, NEB) for PCR reactions.
Data processing
For data analysis, FASTQ read files obtained after sequencing were demultiplexed using the Hi-Seq analysis software (Illumina) and processed using MAGeCK (v.0.5.9.4)69. Raw counts for each sgRNA were generated with MAGeCK ‘count’ module by mapping reads to the mouse metabolic library or the Brie library with non-targeting sgRNAs as the control. The MAGeCK ‘test’ function was used to identify screen hits. For the initial in vitro and in vivo screens, we were able to detect the majority (~99.9% and ~98.8%, respectively) of genes contained in the library from tumour cells (Supplementary Table 1). Total read counts were used for raw count normalization and the secondbest method was used for log2FC quantification. The effects of screen hits were ranked by MAGeCK RRA score.neg (in vitro OT-I treated versus non-treated or C57BL/6 mice + OT-I versus Rag1−/− mice). For the genome-scale secondary genetic interaction screen using the Brie library68, median read counts across all samples were used for normalization, and the ‘mean’ method was used for log2FC quantification and –gene-test-fdr-threshold was set to 1. The significantly enriched or depleted screen hits in sgVdac2-transduced B16-OVA tumour cells were defined as |log2FC| > 0.5 and MAGeCK RRA score < 0.05 (Extended Data Fig. 7a). The targeted genes in the Brie library were further overlapped with genes included in the MitoCarta 3.0 database36 (1,140 for total) to generate a list of 1,032 mitochondria-associated genes (Fig. 4b). The log2FC values and MAGeCK RRA scores of the mitochondria-associated genes in this secondary genetic interaction screen were visualized as a volcano plot by ggplot2 R package (v.3.3.5), with the top 1 and 2 significantly enriched (based on MAGeCK RRA score) mitochondria-associated gene candidates (Casp9 and Bak1) annotated.
Flow cytometry
For analysis of surface markers, cells were first incubated with Fc block (2.4G2, Bio X Cell) for 10 min in PBS containing 2% (w/v) FBS, and then stained with the appropriate antibodies on ice for 30 min. For intracellular cytokine detection, cells were stimulated for 4 h with phorbol 12-myristate-13-acetate (Sigma-Aldrich) plus ionomycin (Sigma-Aldrich) in the presence of monensin (GolgiStop, 554724, BD Biosciences) and stained for surface markers. The cells were fixed and permeabilized using the CytoFix/CytoPerm fixation/permeabilization kit (554774, BD Biosciences) according to the manufacturer’s instructions followed by intracellular cytokine staining using the appropriate antibodies on ice for 30 min. For transcription factor staining, cells were stained for surface markers, followed by fixation and permeabilization using FOXP3/transcription factor staining buffer set (00-5523-00, eBioscience) according to the manufacturer’s instructions and intracellular staining with the appropriate antibodies on ice for 30 min. 7-AAD (A9400, 1:200, Sigma-Aldrich) or fixable viability dye (65-0865-18, 1:1,000, eBioscience) was used for dead cell exclusion. Active caspase-3 staining of control and VDAC2-deficient tumour cells was performed using instructions and reagents from an active caspase-3 apoptosis kit (BD Biosciences). The following antibodies were used: PE–anti-CD45 (1:400, 30-F11, 12-0451-83, eBioscience), FITC–anti-CD45.2 (1:400, 104, 109806, BioLegend), Brilliant Violet 785–anti-CD45.2 (1:400, 104, 109839, BioLegend), Alexa Fluor 700–anti-CD8α (1:400, 53-6.7, 100730, BioLegend), Brilliant Violet 605–anti-CD8α (1:400, 53-6.7, 100743, BioLegend), Alexa Fluor 650–anti-CD4 (1:400, GK1.5, 100469, BioLegend), Brilliant Violet 785–anti-TCRβ (1:400, H57-597, 109249, BioLegend), PE/Dazzle 594–anti-PD-1 (1:400, 29F.1A12, Biolegend,135228), Brilliant Violet 711–anti-B220 (1:400, RA3-6B2, 103255, BioLegend), FITC–anti-CD19 (1:400, eBio1D3, 11-0193-85, eBioscience), PE/Cyanine7–anti-IFNγ (1:200, XMG1.2, 505826, BioLegend), Brilliant Violet 421–anti-TNF (1:200, MP6-XT22, 506328, BioLegend), Alexa Fluor 647–anti-GZMB (1:100, GB11, 515406, BioLegend), FITC–anti-FOXP3 (1:200, FJK-16s, 11-5773-82, eBioscience), BV650–anti-Ki-67 (1:100, B56, 563757, BD Biosciences), Alexa Fluor 647–anti-active caspase-3 (1:100, C92-605, 560626, BD Biosciences), PE–anti-IL-2 (1:200, JES6-5H4, 554428, BD Biosciences). Intratumoural CD8+ T cells were gated as CD45+CD8+TCRβ+; CD4+FOXP3− T cells were gated as CD45+CD4+TCRβ+FOXP3−; CD4+FOXP3+ Treg cells were gated as CD45+CD4+TCRβ+FOXP3+; B cells were gated as CD45+B220+CD19+. Tumour cells were gated as Ametrine+CD45− cells. BD FACSDIva software (v.8) was used to collect flow cytometry data on LSRII, Fortessa or Symphony A3 cytometers (BD Biosciences).
Cytokine-induced cell death assays in vitro
To analyse cytokine-induced cell death, the indicated concentration of IFNγ and/or TNF (554589, BD) or human IFNγ (554616, BD) was added. For cell death inhibition assays, pan-caspase inhibitor emricasan43 (20 μM), ferroptosis inhibitor ferrostatin-170 (Fer-1, 10 μM), necroptosis inhibitor necrostatin-171 (Nec-1, 20 μM) and GSDMD-mediated pyroptosis inhibitor disulfiram72 (20 μM) were used. Tumour cell numbers were quantified at the indicated timepoints by flow cytometry using the cell counting beads. Alternatively, cell death was detected and quantified in real-time using the IncuCyte S3 or IncuCyte SX5 imaging system (Sartorius). In brief, 2 × 104 B16-OVA cells per well were plated into a 48-well plate in RPMI-1640 medium containing 10% FBS, 500 nM propidium iodide (P3566, Invitrogen) or 100 nM SYTOX Deep Red (S11381, Invitrogen) and the indicated concentration of IFNγ (details are provided in the associated figures). Cells were imaged every 1 or 2 h and the PI+ or SYTOX Deep Red+ cells (counted as dead cells) were quantified using the IncuCyte FLR or Zoom software (http://www.essenbioscience.com/en/products/software/) as described previously73. For the LDH-release assay, the cell culture medium was collected at the indicated timepoints and centrifuged at 2,000g for 5 min to obtain the supernatant. LDH release was detected using the CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit (G1780, Promega) according to the manufacturer’s instructions. The absorbance was measured on the VERSAmax Tunable Microplate Reader (Molecular Devices). For annexin-V and 7-AAD staining, tumour cells (including both the adherent and suspension fractions) were washed and resuspended with annexin V binding buffer (00-0055-56, eBioscience) and then stained with APC–anti-annexin V (1:50, BMS306APC-100, Invitrogen) in annexin V binding buffer for 15 min at room temperature. After washing with annexin V binding buffer, the cells were resuspended with 7-AAD working solution (51-65875X, BD) and analysed using flow cytometry.
Cytosolic mtDNA extraction and quantification
B16-OVA cells were cultured in a 10 cm dish and treated with 10 ng ml−1 IFNγ plus pan-caspase inhibitor Q-VD-OPh (40 μM, HY-12305, MedChemExpress) for 24 h, followed by extraction and detection of total DNA and cytosolic DNA as described previously35. In brief, 1 × 107 B16-OVA cells were divided into two equal aliquots. One aliquot was resuspended in 300 μl of 50 mM NaOH and boiled for 60 min to solubilize DNA. Then, 10% volume of 1 M Tris-HCl (pH 7.5) was added to neutralize the pH and then centrifuged at 12,000g for 10 min to pellet intact cells. Moreover, these extracts served as normalization controls for total genomic DNA and mtDNA. The second equal aliquots were resuspended in 300 μl of buffer containing 150 mM NaCl, 50 mM HEPES (pH 7.4), and 20 mg ml−1 digitonin (D141, Sigma-Aldrich). The homogenates were incubated for 15 min on ice to allow selective plasma membrane permeabilization and then sequentially centrifuged at 980g for 3 min for a total of three times to pellet intact cells. Finally, the cytosolic supernatants were transferred to fresh tubes and centrifuged at 16,000g for 10 min to pellet any remaining cellular debris. The cytosolic DNA and total cellular DNA (from whole-cell extracts) were purified using the DNeasy Blood & Tissue Kit (69506, Qiagen). RT–qPCR was performed on both whole-cell extracts and cytosolic fractions using mtDNA primers (mtCytb, mtNd4, mt16S, D-loop1, D-loop2 and D-loop3), and the CT values of whole-cell extracts served as normalization controls for the values of cytosolic fractions (FC = log2−ΔΔCT). A list of the primers used for qPCR analysis is provided in Supplementary Table 11.
Generation of mtDNA-depleted cells
B16-OVA cells were cultured in the presence or absence of 200 ng ml−1 ethidium bromide (EtBr, E7637, Sigma-Aldrich), as described previously44,45, for 6 days. Before IFNγ treatment, the culture medium was replaced, and cells were cultured overnight in the absence of EtBr. To measure the efficiency of mtDNA depletion, total extracts were prepared by resuspending the cells in NaOH 50 mM, incubating at 95 °C for 1 h and neutralizing by adding 10% volume 1 M Tris (pH 7.5). The ratio of mtDNA versus genomic DNA was measured using qPCR .
Immunoprecipitation, subcellular fractionation and immunoblot analysis
For immunoprecipitation, 2 × 106 cells expressing HA-VDAC2 and/or Flag-BAK or Flag-BAX were lysed in ice-cold Pierce IP lysis buffer (87787, Thermo Fisher Scientific) containing protease and phosphatase inhibitor cocktail (78442, Thermo Fisher Scientific) and 1% digitonin (D141, Sigma-Aldrich) with rotation at 4 °C for 30 min. The cell lysate was centrifuged at 13,000g for 10 min at 4 °C, and the supernatant was incubated with anti-Flag (M8823, Sigma-Aldrich) or anti-HA (88836, Thermo Fisher Scientific) magnetic beads at 4 °C for 2 h. The beads were washed three times with ice-cold IP lysis buffer (87787, Thermo Fisher Scientific) and resuspended with 1× complete Laemmle sample buffer (1610747, Bio-Rad).
To detect cytochrome c and SMAC in the subcellular fractions, the mitochondrial and cytosolic fractions were isolated using the Mitochondrial Fractionation Kit (Active Motif) according to the manufacturer’s instructions. In brief, cells were treated with 10 ng ml−1 IFNγ plus 40 μM pan-caspase inhibitor Q-VD-OPh for 24 h and then washed using pre-chilled 1× PBS and centrifuged at 600g for 5 min at 4 °C. The cell pellet was resuspended in 1 ml ice-cold cytosolic buffer and incubated on ice for 15 min, then transferred to a pre-chilled pestle homogenizer. Cells were homogenized using 30–50 strokes with the homogenizer and centrifuged at 800g for 20 min at 4 °C. After centrifugation, the supernatant was transferred to a fresh pre-chilled microcentrifuge tube and centrifuged at 10,000g for 20 min at 4 °C to pellet the mitochondria; the supernatant contained the cytosolic fraction. The mitochondrial pellet was washed once with 100 μl 1× cytosolic buffer and lysed with 100 μl complete mitochondrial buffer on ice for 15 min to obtain the mitochondrial fraction. The cytosolic fraction was transferred to a fresh pre-chilled microcentrifuge tube and centrifuged at 16,000g for 20 min at 4 °C to remove any residual mitochondria.
For chemical cross-linking of cysteines, cells were treated with IFNγ (10 ng ml−1) plus Q-VD-OPh74 (40 μM) for 24 h or ABT-737 (5 mM) + S63845 (5 mM) + Q-VD-OPh75 (40 μM) for 6 h. The mitochondrial fraction was obtained as mentioned before and resuspended in cross-linking buffer (20 mM HEPES/KOH (pH 7.5), 100 mM sucrose, 2.5 mM MgCl2 and 50 mM KCl) containing the fresh added 1,6-bis-maleimidohexane (BMH, 0.5 mM, 13.0 A° linker, Thermo Fisher Scientific) and incubated for 30 min at room temperature. Cross-linking was quenched by addition of reducing buffer (1× complete Laemmle sample buffer with 10% 2-mercaptoethanol, M6250, Sigma-Aldrich), and the samples were analysed by SDS–PAGE.
For immunoblot analysis of tumours treated with isotype or anti-PD-L1 in vivo (as described above), B16-OVA tumour tissues (~100 mg) from tumour-bearing mice were homogenized in 1 ml ice-cold RIPA buffer (89900, Thermo Fisher Scientific) containing protease and phosphatase inhibitor cocktail and homogenized using the Bead Ruptor Elite (OMNI). The lysate was centrifuged at 13,000g for 10 min at 4 °C, and the supernatant was mixed with 4× complete Laemmli Sample Buffer (1610747, Bio-Rad). For immunoblot analysis of cells treated with IFNγ, cisplatin (HY-17394, MCE) or etoposide (HY-13629, MCE) in vitro, cultured cells were directly lysed with 1× complete Laemmli sample buffer. In Extended Data Figs. 8c and 10g, IFNγ-induced expression of BCL-2 family members was analysed in B16-OVA cells without Cas9 expression. All of the protein samples were boiled at 95 °C for 10 min, separated using 4–12% Criterion XT Bis-Tris Protein Gel (3450125, Bio-Rad) and transferred to a PVDF membrane (1620177, Bio-Rad). The membranes were blocked using 5% BSA for 1 h and then incubated overnight with primary antibodies (see below). The membranes were then washed with TBST and then incubated with secondary antibodies for 2 h. After antibody incubation, HRP was activated with Supersignal West Dura Extended Duration Substrate (34075, Thermo Fisher Scientific) and visualized with a chemiluminscent detection system using Amersham Imager 600 (GE Healthcare Life Sciences). The blots were then processed and analysed using Image J. Primary antibodies and dilutions were as follows: anti-VDAC2 (1:1,000, PA5-28106, Invitrogen), anti-BCL-2 (1:1,000, sc-7382, Santa Cruz), anti-MCL-1 (1:1,000, ab32087, Abcam), anti-GSDME (1:1,000, ab215191, Abcam), anti-Flag (1:5,000, F1804, Sigma-Aldrich), anti-BIM (1:1,000, B7929, Sigma-Aldrich); anti-Cas9 (1:5,000, 14697), anti-β-actin (1:5,000, 4970), anti-p-STAT1Y701 (1:1,000, 9167), anti-STAT1 (1:1,000, 14994), anti-caspase-3 (1:1,000, 9662), anti-cleaved caspase-3 (1:1,000, 9661), anti-caspase-7 (1:1,000, 9492), anti-cleaved caspase-7 (1:1,000, 9491), anti-caspase-9 (1:1,000, 9504), anti-APAF-1 (1:1,000, 8969), anti-cGAS (1:1,000, 31659), anti-STING (1:1,000, 13647), anti-p-STINGS365 for mouse cells (1:1,000, 72971), anti-p-STINGS366 for human cells (1:1,000, 19781), anti-TBK1 (1:1,000, 38066), anti-p-TBK1S172 (1:1,000, 5483), anti-IRF3 (1:1,000, 4302), anti-p-IRF3S396 (1:1,000, 29047), anti-MAVS (1:1,000, 4983), anti-BAK (1:1,000, 12105), anti-BAX (1:1,000, 2772), anti-BCL-xL (1:1,000, 2764), anti-BID for mouse cells (1:1,000, 2003), anti-BID for human cells (1:1,000, 2002), anti-PUMA (1:1,000, 98672), anti-HA (1:5,000, 3724), anti-SMAC (1:1,000; 15108), anti-cytochrome c (1:1,000, 4280) and anti-TOMM20 (1:1,000, 42406) (all from Cell Signaling Technology). Secondary antibodies and dilutions were as follows: HRP-conjugated anti-mouse IgG (1:3,000; W4021; Promega) or HRP-conjugated anti-rabbit IgG (1:3,000, W4011, Promega). Densiometric quantification of phosphorylated protein levels was normalized relative to the corresponding total protein, and densiometric quantification of total protein expression was normalized relative to the loading control β-actin or TOMM20 (specifically for Extended Data Fig. 7k). All densiometric quantifications depict the fold changes compared with the relative control (set equal to 1.0) and are shown above the immunoblot image.
MTT assay
To assess tumour cell expansion in vitro, the MTT cell proliferation assay was performed using a commercial kit (30-1010K, ATCC). In brief, 1,000 cells per well B16-OVA-Cas9-sgNTC or B16-OVA-Cas9-sgVdac2 cells were plated onto the 96-well plate on day 0, and the cell number was detected every 24 h according to the manual. Absorbance was measured on the VERSAmax Tunable Microplate Reader (Molecular Devices).
ELISA
For in vivo IFNγ and IFNβ detection, B16-OVA tumour tissues (~200 mg) from tumour-bearing mice were homogenized in 500 μl ice-cold RIPA buffer (89900, Thermo Fisher Scientific) containing protease and phosphatase inhibitor cocktail using Bead Ruptor Elite device (OMNI). The lysate was centrifuged at 13,000g for 10 min at 4 °C, and the supernatant was used for IFNγ and IFNβ enzyme-linked immunosorbent assay (ELISA). For in vitro cultured cells, the culture medium was collected at the indicated timepoints and centrifuged at 13,000g for 10 min at 4 °C to obtain the supernatant. IFNγ and IFNβ was measured by ELISA using the Mouse IFNγ Quantikine ELISA Kit (MIF00-1, R&D systems) or Mouse IFNβ Quantikine ELISA Kit (MIFNB0, R&D systems) according to the manufacturer’s instructions. Absorbance was measured on a VERSAmax Tunable Microplate Reader (Molecular Devices).
Immunostaining and histology analyses
Live-cell imaging was performed using B16-OVA-Cas9-sgNTC or B16-OVA-Cas9-sgVdac2 cells, which were cultured in chambered coverslips (80426, Ibidi). Tumour cells expressing Omi-mCherry were used for determination of MOMP (the pBabe(puro)-Omi-mCherry plasmid expresses fusion protein in the mitochondria intermembrane space, and is released on MOMP39). Time-lapse imaging was performed using the A1RHD25 (Nikon Instruments) resonant scanning confocal equipped with heat and CO2 incubation, and NIS Elements software (64 bit, v.5.30.03). Images were collected with either a ×40/1.3 NA Plan Fluor or 60× 1.3 NA Plan Apo oil objective and 561 nm laser excitation, and acquired with 1,024 × 1,024 with 0.1 μm px−1 resolution.
mtDNA imaging was performed using control (sgNTC) and VDAC2-deficient B16-OVA cells with or without 10 ng ml−1 IFNγ plus 40 μM pan-caspase inhibitor Q-VD-OPh treatment for 0 to 24 h as indicated in figures. All of the cells were cultured in chambered coverslips (80426, Ibidi), fixed with 2% paraformaldehyde for 10 min at room temperature, and then treated with 0.1% Triton-100 for permeabilization. Cells were blocked with PBS containing 1% bovine serum albumin and 5% normal goat serum before addition of anti-dsDNA (1 μg ml−1; MAB030, Millipore-Sigma), anti-TOMM20 (1 μg ml−1; 186735, Abcam) or anti-HA (1 μg ml−1; 2367, Cell Signaling Technology) antibodies, followed by detection with donkey anti-mouse (1:500, A32773, Thermo Fisher Scientific) and donkey anti-rabbit (1:500, A32795, Thermo Fisher Scientific) secondary antibodies. Images were acquired using the A1RHD25 (Nikon Instruments) resonance scanning confocal microscope using a ×40/1.3 NA Plan Fluor oil objective, 1,024 × 1,024 and 0.1 μm px−1 resolution, 561 nm and 640 nm laser lines. Images were deconvolved using NIS Elements (64 bit, v.5.30.03) and analysed using Imaris software (Bitplane, v.9.5.1×64). For histology analyses, mouse tissues were fixed by 10% (v/v) neutral buffered formalin solution, embedded in paraffin, sectioned and stained with haematoxylin and eosin.
RNA isolation and gene expression profiling
Cells were lysed with Buffer RLT in the RNeasy Micro Kit (74004, Qiagen), and total RNA was extracted according to the manufacturer’s instructions. Then, 1 μg total RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (4368814, Applied Biosystems). Diluted cDNA was subjected to RT–qPCR reactions containing Power SYBR Green PCR Master Mix (4367659, Applied Biosystems) and gene-specific primers. The reactions were performed in a QuantStudio7 Flex Real-Time PCR System (Applied Biosystems). Actb was used as the housekeeping control. A list of the primers is provided in Supplementary Table 11.
Microarray transcriptome analyses
For microarray analysis, to analyse the gene expression of tumour cells after treatment with OT-I cells, control or VDAC2-deficient B16-OVA tumour cells were treated with OT-I cells for 24 h, and the remaining tumour cells were sorted for RNA extraction (n = 4 replicates each group). To compare the differently expressed genes in various groups (control, VDAC2-deficient; BAK-deficient; VDAC2 and BAK co-deficient; STING-deficient; VDAC2 and STING co-deficient; APAF-1-deficient; VDAC2 and APAF-1 co-deficient; BIM and BID co-deficient; or VDAC2, BIM and BID co-deficient) of B16-OVA tumour cells, tumour cells were treated with or without IFNγ at 10 ng ml−1 for 24 h in vitro, and the adherent tumour cell fraction was collected for RNA extraction (3 or 4 replicates for each group). To analyse the gene expression of LoVo cells after treatment with IFNγ, control or VDAC2-deficient LoVo tumour cells were treated with human IFNγ for 48 h, and the remaining tumour cells were collected for RNA extraction (n = 3 replicates each group). After RNA extraction and purification, 125 ng RNA was used to profile with Affymetrix mouse or human Clariom S assay. The expression signals were analysed using Affymetrix Expression Console (v.1.4.1), followed by differential expression analysis performed using R package limma (v.3.34.9). All of the plots were generated using R packages ggplot2 and ComplexHeatmap (v.2.6.2). Volcano plots depicting log2FC and –log10(P value) were plotted, differentially expressed genes were defined by |log2(FC)| > 0.5; P < 0.05, and the top enriched genes were highlighted.
For GSEA, the preprocessed expression dataset and gene sets were input into GSEA (v.4.3.2), and gene sets were ranked based on their enrichment scores calculated using the two-tailed Kolmogorov–Smirnov test. A ranked GSEA was conducted using the default settings except for ‘Permutation type’, which was set to ‘gene_set’. Hallmark signatures from the Molecular Signatures Database were used (MSigDB; https://www.broadinstitute.org/gsea/msigdb/; v.7.4). For IPA analysis, we used the commercial QIAGEN’s Ingenuity Pathway Analysis (IPA, QIAGEN; www.qiagen.com/ingenuity, v.01-23-01) software for upstream regulatory analysis of differentially expressed genes identified in VDAC2-deficient versus WT B16-OVA cells at 16 h after IFNγ treatment, or at 24 h after OT-I cell treatment. For upstream regulatory analysis, we used |log2FC| > 0.4 as the threshold to select 249 upregulated genes and 262 downregulated genes as the input. In the microarray dataset profiling tumour cells with single deletion or co-deletion of VDAC2 and STING treated with IFNγ for 24 h, differentially expressed genes (sgVdac2 versus sgNTC tumour cells, log2FC > 0.5, P < 0.05) were first intersected with genes in Hallmark IFNα and IFNγ response pathways to obtain a list of 15 IFN-response genes. The relative expression of these 15 IFN-response genes were then visualized by heat map in the four genotypes (sgNTC + sgNTC, sgVdac2 + sgNTC, sgNTC + sgSting1 and sgVdac2 + sgSting1).
scRNA-seq and data analysis
Library preparation
For scRNA-seq analysis, wild-type mice were challenged with control or VDAC2-deficient B16-OVA tumour cells. CD45+ immune cells and Ametrine+ CD45– tumour cells in the tumour tissues were sorted on day 14 after tumour inoculation and mixed at a 3:1 ratio (n = 2 biological replicates per genotype). The cell mixture was centrifuged at 2,000 rpm for 5 min and then resuspended in 1× PBS (Thermo Fisher Scientific) plus 0.04% BSA (Amresco) with a final concentration of 1 × 106 cells per ml. The single-cell suspensions were loaded onto the Chromium Controller and encapsulated into droplets. The Chromium Next GEM Single Cell 5′ (version 2) and Gel Bead Kit (10x Genomics) was used for the library preparation according to the manufacturer’s instructions. The final libraries were quality-checked using the 2100 Bioanalyzer (Agilent Technologies). The resulting libraries were sequenced on the NovaSeq (Illumina) system with paired-end reads of 26 cycles for read 1 and 90 cycles for read 2 and 10 cycles for index 1 and 2 separately). An average of 500 million reads per sample was obtained.
Data processing and quality control
The Cell Ranger (v.6.0.0) Single-Cell software suite (10x Genomics) was used to process the scRNA-seq FASTQ files. The ‘cellranger count’ command was performed to align the raw FASTQ files to the mm10 mouse reference genome and summarize the data into matrices that describe gene read counts per cell. For the datasets with matched TCR-seq data, the ‘.vdj’ command was used to generate a count matrix, which, after filtering, was used for downstream analyses.
For gene expression sequencing, the filtered count matrices were read into the R package Seurat (v.4.1). The samples (control and VDAC2-deficient tumours with corresponding CD45+ immune cells) were merged into a single Seurat object for consistent filtering, and features detected in fewer than three cells were removed from the dataset. Cells with abnormally low features or unique molecular identifier (UMI) counts or high mitochondrial read percentages (potentially dead or damaged cells) were removed. Cells with abnormally high UMI counts (potentially multiple cells in a single droplet) were also removed. Finally, any remaining multiplets expressing mutually exclusive marker genes were removed. After filtering, 17,344 cells were retained with an average of 4,064 genes per cell (UMI median: 11,006; range: 500–149,945). After quality control, libraries were normalized with the NormalizeData function (scale.factor = 1 × 106) in the Seurat R package.
Cluster annotation and data visualization
Normalized and filtered data were processed using the standard Seurat pipeline. UMAP dimensionality reduction was used for visualization, and Seurat’s FindClusters function was used to separate cells into unsupervised clusters. Cell types in clusters were defined using the following marker genes: B cells (Cd19+), CD8+ T cells (Cd3d+Cd8a+), CD4+ T cells (Cd3d+Cd4+Foxp3–), cDC1 (Xcr1+), cDC2 (Cd209a+), macrophages (Adgre1+), mregDC (Ccr7+, Cd200+, Fscn1+), neutrophils (Hdc+), NK cells (Ncr1+), pDC (Siglech+), Treg cells (Foxp3+) and tumour cells (Cd45–). Tumour cells were further verified based on the inferred presence of somatic copy-number alterations using inferCNV (v.1.3.5). For the CreateInfercnvObject() function, we used CD8+ T cells as a negative control group. For the run() function, we used the following parameter values: cutoff=0.1, cluster_by_groups=TRUE, denoise=TRUE, HMM=TRUE. CD8+ T cells were further clustered into stem-like (Tcf7+Havcr2–), effector-like (Tcf7–Havcr2+Pdcd1+ToxintMki67+) and terminally differentiated (Tcf7−Havcr2+Pdcd1+ToxhighCd38+Cd101+Mki67−) CD8+ T cells based on the expression of the indicated markers.
TCR data analysis and visualization
For TCR-seq, filtered contig annotation matrices from the Cell Ranger output were loaded into R. Annotation and quantification of TCR clonotypes were processed with the scRepertoire package (v.1.3.5). Clonal frequencies in CD8+ T cells (Cd3d+Cd8a+) were categorized as follows: NA (0), single (1), small (2–5), medium (6–20), large (21–100) and hyper (101–500). Clonal expansion bar plots were generated using the ggplot2 R package. The clonotypic information was integrated to the Seurat object using the combineExpression() function, with the cloneTypes variable set to cloneTypes=c((Single=1, Small=5, Medium=20, Large=100, Hyperexpanded=500). The cell frequencies of different clonetype sizes were further plotted as a stacked bar plot by ggplot2 R package. Cells were considered clonally expanded if the clonotype size was greater than 1 and non-expanded if the clonotype size was equal to 1.
GSEA and signature curation
For scRNA-seq analysis, nonparametric Wilcoxon rank-sum tests were used to compare the gene expression of cells between two genotypes (sgVdac2 versus sgNTC) and then genes in each comparison were ranked on the basis of their log2FC. To identify the enriched pathways, pre-ranked GSEA (an analysis of GSEA against a user-supplied, ranked list of genes) was then performed as previously described76 against gene sets from the Hallmark collection from the Molecular Signatures Database (mSigDB) (https://www.broadinstitute.org/gsea/msigdb/, v.7.4). For CD8+ T cells, gene signatures of ‘early activation’ and ‘effector/cytokine’ were curated using a previous publication77, with their activity scores calculated using the AddModuleScore() function in Seurat R package. The VDAC2-suppressed gene signature was generated by defining the upregulated genes (log2FC > 0.7, FDR < 0.05) in VDAC2-deficient versus control tumour cells based on the scRNA-seq data. A total of 26 human homologues of genes included in the list of VDAC2-suppressed genes was used for TGCA analysis (Supplementary Table 4). Specifically, to assess whether expression of VDAC2-suppressed genes is associated with the survival outcome of patients with cancer, we assigned patients with cutaneous melanoma from the TCGA SKCM cohort to high or low groups based on the median value of expression of VDAC2-suppressed genes and performed overall survival analysis using the interactive web-based tool GEPIA v.2 (http://gepia2.cancer-pku.cn/#survival)78. The survival results were displayed using Kaplan–Meier curves. P value = 0.05 was used as the threshold of statistical significance. The dotted lines in Extended Data Fig. 4w indicate the 95% confidence interval. To assess whether the expression of VDAC2-suppressed genes is associated with the survival of patients with cancer with anti-PD-1 therapy, the association between expression of VDAC2-suppressed genes and the overall survival in patients treated with anti-PD-1 were tested using Cox proportional hazards regression analysis in melanoma using TIDE (http://tide.dfci.harvard.edu/login/)79,80. The P values were generated using the two-sided Wald test in the Cox proportional hazards regression. Patient groups with high or low expression of VDAC2-suppressed genes were stratified by the median value of the signature expressing.
ATAC–seq sample preparation and analysis
C57BL/6 mice were s.c. implanted with 1 × 106 control or VDAC2-deficient or control B16-OVA cells. At day 15 after tumour inoculation, control or VDAC2-deficient B16-OVA cells were sort-purified for ATAC–seq analysis (n = 4 biological replicates per group). For ATAC–seq profiling of CD8+ T cells from tumours, PD-1–CD8+ T cells and PD-1+CD8+ T cells were sorted from control or VDAC2-deficient B16-OVA tumours at day 14 after tumour inoculation (n = 4 biological replicates per group). Sorted cells were incubated in 50 μl ATAC–seq lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2 and 0.1% IGEPAL CA-630) for 10 min on ice. The resulting nuclei were pelleted at 500g at 4 °C for 10 min. The supernatant was discarded. The pellet was resuspended in 50 μl transposase reaction mix (25 μl 2× TD buffer, 22.5 μl nuclease-free water and 2.5 μl transposase) and incubated at 37 °C for 30 min to allow tagmentation. The DNA was cleaned up using a Qiagen MinElute kit. The barcoding reaction of the tagmented DNA was run by a NEBNext HiFi kit and amplified for five cycles as previously described using the same primers. The optimal cycle numbers were determined from 5 μl (of 50 μl) from the previous reaction mix using KAPA SYBRFast (Kapa Biosystems) and a 20-cycle amplification on the Applied Biosystems 7900HT system. The remaining 45 μl of PCR reaction was amplified in the same reaction mix using the optimal cycle number.
ATAC–seq data analysis
Paired-end reads (2 × 50 bp) generated from NovaSeq were trimmed to remove Nextera adaptors using trimmomatic (v.0.36) in paired-end mode with the parameters LEADING:10, TRAILING:10, SLIDINGWINDOW:4:18 and MINLEN:25. These reads were then aligned to the mm10 mouse genome by BWA (v.0.7.16, default settings). Duplicated reads were flagged using Picard (v.2.9.4) and only unique, properly paired reads were retained using SAMtools (with the parameters ‘-q 1 -F 1804’; v.1.9). After adjustment of Tn5 transposase shift (with reads shifted +4 bp on the sense strand and −5 bp on the antisense strand), the fragments were divided into nucleosome-free, mononucleosome, dinucleosome and trinucleosome categories based on size as previously described29. Bigwig files were created using the centre 80 bp of each fragment, normalized to 30 × 106 nucleosome-free reads. All of the samples contained approximately 2 × 108 nucleosome-free reads, reflecting high data quality. Next, peaks in nucleosome-free regions were identified using MACS2 (v.2.1.1.20160309, with the default parameters with ‘–extsize 200–nomodel’). To enhance the reproducibility, peaks were retained only if they passed a stricter threshold (MACS2 –q 0.05). Group consensus peaks were formed by retaining peaks present in at least 50% of replicates and discarding the rest. The reproducible peaks were further merged between samples if they overlapped by at least 100 bp and nucleosome-free reads from each sample were counted using bedtools (v.2.25.0).
To identify the differentially accessible open chromatin regions, the raw nucleosome-free read was first normalized as counts per million followed by differential accessibility analysis by implementation of the negative binomial model in the DESeq2 R package (v.1.43.5). P < 0.05, |log2(FC)| > 0.4 were used as cut-off values for more-accessible or less-accessible regions in B16-OVA tumour cells. P < 0.05, |log2FC| > 0.5 were used as cut-off values for more-accessible or less-accessible regions in CD8+ T cells from tumours. Principal component analysis (PCA) was performed using the function prcomp in R. Functional peak set enrichment was then performed using MSigDB, Hallmark, C2 and C5 collection for those differentially accessible genes. For motif enrichment analysis, 1,000 unchanged regions (log2FC < 0.05 and P > 0.5) were selected as control regions for each comparison. FIMO from MEME suite (v.4.11.3, ‘–thresh 1e-4–motif-pseudo 0.0001’) was used for scanning motifs (TRANSFAC database release 2019, only included Vertebrata) matches in the nucleosome-free regions, and two-tailed Fisher’s exact tests were used to determine whether a motif was significantly enriched in differentially accessible regions compared with the control regions.
Analysis of public transcriptomics datasets
To examine the expression of VDAC1, VDAC2 and VDAC3 in tumour cells and other immune cells from the TME, the human melanoma (GEO: GSE215121)81, human non-small cell lung cancer (NSCLC) (GEO: GSE148071)82 and mouse tumour scRNA-seq (GEO: GSE121861)83 datasets were analysed using Seurat. For the analysis of the human melanoma dataset, we used the provided read count matrix in GSE215121. After creating the Seurat object, we pooled the 11 samples using the merge() function. Cells were initially quality filtered based on the percentage of mitochondrial reads < 10% (to remove dead cells) and the number of detected RNA features (gene number <5,000 and UMI < 40,000 to remove potential doublets), and then the data were processed using the standard Seurat pipeline. Cell types were determined based on the indicated markers: T cells (CD3D+CD3E+), B cells (MS4A1+CD79A+), NK cells (FGFBP2+KLRD1+), combined monocytes and macrophages (LYZ+CD68+CD14+), melanoma cells (MLANA+PMEL+MITF+DCT+), endothelial cells (VWF+PECAM1+) and fibroblasts (COL1A1+COL3A1+). For the analysis of the human NSCLC dataset, we used the prefiltered read count matrix provided in GSE148071. After creating the Seurat object, we pooled the 42 samples using the merge() function. The data were then processed using the standard Seurat pipeline. Cell types were defined using the marker provided in the paper82: endothelial cells (CLDN5+VWF+PECAM1+), epithelial cells (CAPS+SNTN+), alveolar cells, (CLDN18+AQP4+FLOR1+), fibroblasts (COL1A1+COL1A2+DCN+), T cells (CD2+CD3D+CD3E+CD3G+), B cells (CD79A+CD79B+), myeloid cells (CD14+LYZ+), neutrophils (CSF3R+S100A8+S100A9+), follicular dendritic cells (FDCSP+), mast cells (GATA2+TPSAB1+TPSB2+) and cancer cells (EPCAM+). For both the human melanoma and NSCLC tumour datasets, the expression of VDAC1, VDAC2 and VDAC3 in different cell types was visualized by DotPlot() function from the Seurat package. For the mouse tumour scRNA-seq dataset, the expression of Vdac1, Vdac2 and Vdac3 in the indicated cell types from GSE121861 was also visualized using the DotPlot() function from the Seurat package.
The perturbation effects of VDAC2 among tumour lines in DepMap were visualized by Chronos dependency scores. The perturbation effects of VDAC2 in human melanoma cells treated with TILs was visualized by comparing ‘neg | score’ and ‘neg | rank’ columns in the comparison of cells treated with TILs at 1:1 ratio versus control cells on day 1722. The Pearson correlation between the VDAC2 gene and the 18-gene tumour inflammation signature (including HLA-E, NKG7, CD8A, PSMB10, HLA-DQA1, HLA-DRB1, CMKLR1, CCL5, CXCL9, CD27, CXCR6, IDO1, STAT1, TIGIT, LAG3, CD274, PDCD1LG2 and CD276)84, CCL5 or CD3D85 was calculated in each tumour type from TCGA database. Skin cutaneous melanoma and NSCLC tumour RNA-seq data from TCGA were deconvoluted by CIBERSORTx (v.1.05)86 to determine the fraction of CD4+ and CD8+ T cells.
Statistical analysis for biological experiments
For biological experiment (non-omics) analyses, data were analysed by Prism v.10 software (GraphPad) using two-tailed unpaired Student’s t-tests, one-way ANOVA or two-way ANOVA, as indicated in the figure legends. The Mantel–Cox test was used for comparing mouse survival curves. Two-tailed Wilcoxon rank-sum tests were applied for activity score or expression analysis of scRNA-seq data. P < 0.05 was considered statistically significant, with the exact P values provided in the source data that accompany this Article. In all bar plots, data are mean ± s.e.m.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.