For seven years, British neuroscientist Luke Bashford trained as a postdoc in the United States, working on brain–computer interfaces (BCIs) — systems that directly link brain activity to external devices. By recording and decoding electrical signals from the brain to generate computer commands, BCIs allow people with limited movement to use their thoughts to control technologies such as smartphones, computers, wheelchairs and robotic arms. Unlike non-invasive BCIs — wearable devices such as caps or headbands that attach electrodes to the outside of the head — the implantable BCIs that Bashford works on require surgery to place the electrodes on or inside the brain to access more reliable and information-rich signals.
A lot of academic research — and most commercial investment — is focused on implantable BCIs, because their potential to provide high-performance assistive interfaces is stronger than their non-invasive counterparts. But implanting electrodes directly in the brain comes with obvious risks, so implantable BCI trials are tightly controlled by regulators. The most advanced implantable BCIs in development remain in early-stage clinical trials, and no such system has been approved for clinical use anywhere in the world.
Nature Index 2024 Neuroscience
The United States took an early lead on regulating implantable BCI, which has made it an attractive place for researchers such as Bashford to work. Since the first volunteer received an implantable BCI in California in 2004, most of the roughly 60 long-term recipients have been based in the United States. All of the world’s most established implantable BCI companies are based there, too. The US Food and Drug Agency (FDA), which oversees all US implantable BCI trials, is now very familiar with the technology, says Bashford. “You make a query, and they come back with a very nice framework of ‘Here’s what’s got to be done and how and why’.”
The dominance of the United States raises concerns about the potential for unequal access to implantable BCI technologies as they move from the laboratory to the clinic. When Bashford left his position at the University of California, Los Angeles, in 2023 to move to the University of Newcastle, in the United Kingdom, he realized how much work it would take to achieve his goal of running the country’s first clinical trials of implantable BCIs. When it comes to UK regulators, “there’s definitely an appetite for it”, he says. But the country’s inexperience with the technology makes getting approval for a clinical trial a lengthy process.
Bashford co-founded a National Consortium for Neurotechnology Regulation (NCNR) in February with a group of UK-based researchers and companies to help address the problem. By forging greater connections between academics, clinicians, industry, regulators and policymakers, the NCNR aims to set guidelines for human neurotechnology trials, which it hopes will ultimately accelerate patient access to such devices on the UK’s National Health Service.
Global private investment in BCIs and other neurotechnologies were worth an estimated US$7.3 billion in 2020 — a 22-fold increase from 2010. As research in this area becomes more widely distributed, national regulatory bodies are likely to play a key role in how trials progress and products develop, says Tim Denison, NCNR member and neurotechnology engineer at the University of Oxford, in the UK.
Global competition
Ruten, a company that makes implantable BCIs, has headquarters in both the United States and Japan. As a result, co-founder Kazutaka Takahashi has first-hand experience of the regulatory differences between the two countries. Japan, he says, lacks the expertise needed to evaluate new devices through the country’s Pharmaceuticals and Medical Devices Agency. “They’re still trying to come up with standards to be enforced in clinical trials,” he says. In the US, by contrast, the FDA has established protocols that it applies to initial feasibility trials of implantable BCIs. Ruten is working on implantable BCI-based therapies for paralysed people who have trouble swallowing. Almost certainly, any human trials of the device will be based in the United States, says Takahashi, following pathways set by the FDA.
Likewise, several of Europe’s top emerging neurotechnology companies are developing their implantable products in the United States, taking FDA pathways towards clinical approval and the market. Carolina Aguilar, chief executive of INBRAIN Neuroelectronics, says that for the company’s implantable epilepsy monitor, which requires similar implantation procedures to BCIs, going to the United States first is an obvious move. The device is designed to pinpoint where a patient’s epileptic activity originates and so needs to be implanted for only a month. In the United States, this qualifies it for non-implant status, which requires only animal testing to get FDA approval for clinical use, says Aguilar. In Europe, the device is classified as a chronic implant, which requires human testing for approval by the European Union’s Medical Device Regulation (MDR) agency.
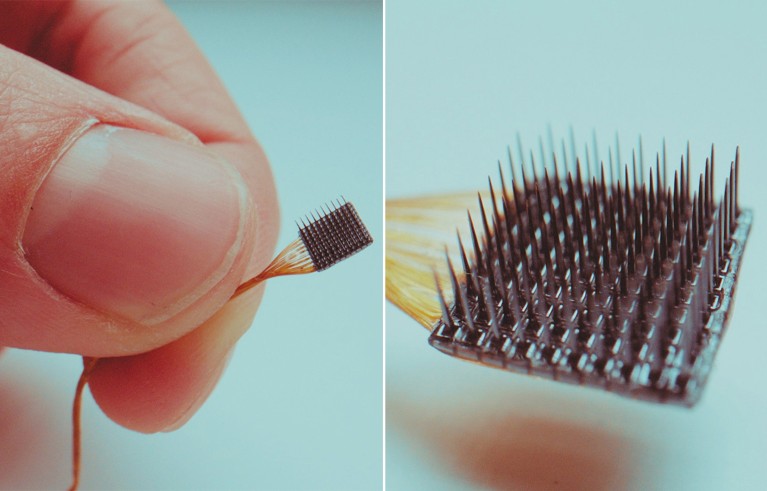
The Utah Array, an implantable BCI developed by Blackrock Neurotech in Utah, can stimulate individual neurons or groups of neurons.Credit: Blackrock Neurotech
The relative ease with which researchers and companies can develop their products in the United States is a problem, says Takahashi, because early recipients of implantable BCIs should be more globally representative. He also worries about US health-care systems and insurers having an outsized influence on the industry, meaning only products that align with what they are willing to cover would make it to market. “If there’s only one country doing this, that’s bad,” says Takahashi.
US dominance in the area has a lot to do with the large investments that have come from government and venture-capital firms over the past 25 years, says Matt Angle, chief executive of Paradromics, a BCI company based in Austin, Texas. Today, the combination of an established regulatory landscape and the world’s most valuable medical-device market — worth an estimated US$180 billion last year — appeals to start-ups from all over the world. “The regulatory pathway for these kinds of devices is better defined and the wheels are better greased in the US than in Europe,” Angle says.
In addition to initiatives launched by the FDA in recent years, such as Early Feasibility Studies, which introduced exemptions for small exploratory studies in 2013, and its Breakthrough Devices Program, launched in 2016 to accelerate communication between developers and FDA officials, Angle also thinks a surge of new recruits at the agency has been a game-changer. “As recently as 2010, I would say the regulatory process was seen as an adversarial process, like a courtroom proceeding,” he says. “In 2024, it’s seen as a collaborative process. If you hadn’t had an influx of a new generation of people at the FDA, none of this would have worked.”
Vikash Gilja, chief scientific officer at Paradromics, adds that many newer recruits at the FDA who deal with neurotechnology were once researchers with direct experience in the field. “They can act as really impactful translators between the medical device innovators and the FDA,” says Gilja. He points to the Implantable BCI Collaborative Community, established by the FDA this year to bring together government regulators, companies, academics and patient advocates, as an important step in advancing implantable BCI-related policies.
Patient benefits
Whether the United States will remain the favoured route for international companies is uncertain. INBRAIN is seeking approval to run human trials of its epilepsy monitor in both the United States and the United Kingdom, the latter through its Medicines and Healthcare products Regulatory Agency (MHRA). Although the MHRA required a “huge amount of work” as part of its application process, the organization has been “super-supportive”, says Aguilar. She is also optimistic about how the EU’s MDR is updating its regulatory pathways and says INBRAIN intends to trial a speech-decoding BCI — an implantable device that records speech-related neural activity in patients — in Europe. “We’re talking to many investigators who want to make it happen from the European perspective,” says Aguilar. “Europe is waking up, because they have to — because they have seen the advantages of the FDA.”
Patient benefit is another major factor in how countries are choosing to regulate implantable BCIs. Denison says the globally accepted standards for keeping research participants safe means that no country’s approach is more dangerous than another’s. But regulators can differ in how they view the benefits of exploratory science to individual patients versus the potential clinical benefit for all future users. “Each country has a slightly different perspective on what they think is acceptable, in terms of the trade-offs,” he says.
Having moved from the United States to the United Kingdom, Bashford is experiencing this tension. In addition to not having the kinds of exploratory study programmes that the FDA runs, the UK’s apparent reluctance to have volunteers participate in early-stage medical-device research speaks to cultural differences between British and US regulators, says Bashford. In the United States, there is a broader view of patient benefit, where participating in research “can just improve someone’s outlook and give them a sense of purpose, where they might otherwise just be left in palliative care”, he says. Denison adds that compared with the FDA, the MHRA asks much earlier in the process how a device will help future users and how that can be assessed from the outset. “I like the MHRA approach because it really keeps me very focused on the translation stuff,” he says.
As one company draws closer to its goal of taking an implantable BCI to market, questions about patient benefit will need to be addressed. Synchron, a New York-based company founded on technology originally developed in Australia, has produced a device that allows recipients to control a smartphone using their thoughts. The company is in discussions with the FDA about what a large human trial must show in order to gain approval to go to market. “This is one of the biggest questions right now: how do we think about clinical endpoints in a pivotal study?” says Angle.
For example, should an implantable BCI be assessed on how efficiently signals are transferred from a user’s brain to a computer interface, or by how much it subjectively improves the user’s wellbeing? Or, perhaps more likely, will it be measured by how well the computer or other external device is controlled? The bar must be set just right, says Angle, to ensure that implantable BCIs can leave the lab and impart meaningful benefits to patients.



