Mice
Mice were maintained in specific-pathogen-free conditions at a temperature between 18 °C and 23 °C with 40–60% humidity and a 12 h–12 h light–dark cycle in accordance with the Institutional Animal Care and Use Committee of the University of California, San Diego (UCSD). All mice were of C57BL/6J background and bred at the UCSD or purchased from The Jackson Laboratory. R26creERT2 (stock no. 008463, The Jackson Laboratory), Tgfbr2fl/fl (stock no. 012603, The Jackson Laboratory), P14 Cas9–eGFP (stock no. 026179, The Jackson Laboratory), P14 and CD45.1 congenic mice were bred in-house. Male recipient mice were used for adoptive transfer experiments, and females were used as P14 CD8 T cell donors. In the spatial pooled CRISPR knockout experiment, a male was used as a donor. To delete floxed alleles using Cre-ERT2, 1 mg of tamoxifen (Cayman Chemical) emulsified in 100 µl of sunflower seed oil (Sigma-Aldrich) was administered by intraperitoneal injection for five consecutive days to P14 R26creERT2Tgfbr2WT (WT) and P14 R26creERT2Tgfbr2fl/fl (TGFβRII knockout) mice before P14 CD8 T cell isolation. All mice were between 1.5 and 6 months old at the time of infection and randomly assigned to experimental groups. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications from our laboratory and others. No blinding was performed during mouse experiments. Investigators were not blinded to group allocation during data collection and/or analysis. Mice were fed ad libitum for the specified amount of time. All animal studies were approved by the Institutional Animal Care and Use Committee at the UCSD and performed in accordance with UCSD guidelines.
Adoptive cell transfer of naive P14 CD8 T cells and LCMV infection in mice
A total of 5 × 104 female naive P14 CD8 T cells isolated by negative enrichment using magnetic activated cell sorting (MACS) and resuspended in PBS were transferred intravenously into congenically distinct male recipient mice. Recipient mice were subsequently infected intraperitoneally with 2 × 105 plaque-forming units of the Armstrong strain of LCMV.
Preparation of single-cell suspensions for flow cytometry
The isolation of CD8 T cells was performed as described previously38. SI intraepithelial lymphocytes and lamina propria lymphocytes were prepared by removing Peyer’s patches and the luminal contents from the entire SI. The SI was then cut longitudinally into 1 cm pieces and incubated at 37 °C for 30 min in HBSS with 2.1 mg ml−1 sodium bicarbonate, 2.4 mg ml−1 HEPES, 8% bovine growth serum and 0.154 mg ml−1 dithioerythritol (EMD Millipore). The samples were passed through a 70-µm cell strainer, and the supernatant constituted the intraepithelial lymphocyte compartment of the SI. The remaining tissue fragments of the SI were further incubated in RPMI with 1.2 mg ml−1 HEPES, 292 µg ml−1 l-glutamine, 1 mM MgCl2, 1 mM CaCl2, 5% fetal bovine serum (FBS) and 100 U ml−1 collagenase (Worthington) at 37 °C for 30 min. After enzymatic incubation, samples were filtered through a 70-μm nylon cell strainer (Falcon). Tissue preparations were separated on a 44%/67% Percoll density gradient.
The following antibodies were used for flow cytometry: CD3 (PE clone 145-2C11, eBioscience 12-0031-83, 1:200 dilution), TCR αβ (APC clone H57-597, eBioscience 17-5961-83, 1:200 dilution), NK1.1 (FITC clone PK136, eBioscience 11-5941-81, 1:400 dilution), CD19 (PerCP-Cy5.5 clone eBio1D3, eBioscience 45-0193-82, 1:200 dilution), CD8b (BV421 clone H35-17.2, eBioscience 48-0083-82, 1:400 dilution), CD45.1 (BV510 clone A20, BioLegend 110741, 1:200 dilution), TCR γδ (BV711 clone GL3, BioLegend 118149, 1:200 dilution), CD4 (BV786 clone GK1.5, BioLegend 100453, 1:400 dilution), CD8a (PE-Cy7 clone 53-6.7, eBioscience 25-0081-82, 1:400 dilution), fixable viability dye (APC-Cy7 eBioscience 65-0865-14, 1:1,000), CD11b (PE clone M1/70, eBioscience 12-0112-82, 1:200 dilution), CD11c (APC clone N418, BioLegend 117310, 1:200 dilution), Ly6C (FITC clone AL-21, BD 553104, 1:200 dilution), Ly6G (PerCP-Cy5.5 clone 1A8, BioLegend 127615, 1:200 dilution), XCR1 (BV421 clone ZET, BioLegend 148216, 1:200 dilution), CD45 (BV510 clone 30-F11, BD 561487, 1:200 dilution), F4/80 (BV711 clone BM8, BioLegend 123147, 1:200 dilution), MHC II (BV786 clone M5/114.15.2, BioLegend 107645, 1:200 dilution) and B220 (PE-Cy7 clone RA3-6 B2, BioLegend 103222, 1:200 dilution).
Sample preparation for histology of the mouse SI
For fresh frozen samples, mouse SIs were collected, retaining the proximal–distal orientation. After discarding the first 3 cm proximal section, approximately 10 cm of mouse proximal SI (containing duodenum and proximal jejunum) was rinsed in ice-cold PBS and the lumen contents were flushed with 20 ml of ice-cold PBS using a gavage syringe. The SI was then loaded onto a 3.25-mm-diameter knitting needle premoistened with cold PBS and placed directly on thick blotting paper. The Mouse Intestinal Slicing Tool39 was used as a guide for the scalpel to cut the intestine longitudinally along the knitting needle. The Mouse Intestinal Slicing Tool and needle were removed, and the SI was spread open and rolled using a wood autoclaved round toothpick, embedded in OCT in plastic moulds, frozen in dry ice(Tissue-Tek Cryomold) and kept at −80 °C until cryosectioning. For fixed frozen samples, the opened cleaned SIs were fastened to blotting paper by minutien pins in each corner and fixed in 4% paraformaldehyde solution in PBS at 4 °C for 16 h, followed by incubation in 70% ethanol at 4 °C for a minimum of 3 h. The SI samples were then rolled using a wood autoclaved round toothpick, snap-frozen in OCT in plastic moulds for cryosection (Tissue-Tek Cryomold) and kept at −80 °C until processing. For formalin-fixed paraffin-embedded (FFPE) samples, fixed SIs were rolled, mounted on 2% agar round moulds and placed on histology cassettes for paraffin embedding.
Histology and immunofluorescence staining of fresh frozen mouse tissues
After OCT block equilibration at −20 °C, 10-mm slices were obtained using a cryostat, mounted on glass slides, dried for 20 min at −20 °C and fixed in ice-cold acetone at −20 °C for 20 min. After fixation, the slides were dried briefly at room temperature and stored at −80 °C until stained or used immediately. For staining, the slides were equilibrated at room temperature, washed in 4 °C PBS twice for 5 min, blocked in serum-free blocking reagent overnight (Dako) at 4 °C, followed by staining with CD45.1–AF594 (BioLegend, clone A20, 110756, 1:50 dilution) and E-cadherin-APC (BioLegend, clone DECMA-1, 147312, 1:200 dilution) and CD8a–FITC (BioLegend, clone 53-6.7, 35-0081-U500, 1:50 dilution) diluted in antibody diluent solution (Dako, S080983-2) overnight at 4 °C, stained with DAPI and mounted with coverslips using Vectashield Vibrance Antifade mounting medium (VectorLabs, H-1700). Images were acquired on an Olympus VS200 Slide Scanner (UCSD Microscopy Core) or on a Zeiss LSM700 confocal microscope. P14 CD8 T cell distances for IMAP representation over time were quantified using a groovy script on QuPath (https://github.com/Goldrathlab/Spatial-TRM-paper).
Single-nucleus RNA-seq of mouse SI
Female CD45.1+CD8+ P14 T cells were adoptively transferred into male CD45.2+ recipients (1 × 105 cells per mouse) 30 min before infection with the Armstrong strain of LCMV. At 28 d.p.i., the mice were euthanized and the SI was dissected, flayed and washed in cold PBS; Peyer’s patches were excised from the SI. The SI was divided into three equal sections designated as the proximal, middle and distal SI. Tissue sections were cut into pieces of approximately 3 mm and flash-frozen in liquid nitrogen for 2 min. Nucleus isolation was performed with 10x Genomics Chromium Nuclei Isolation Kit per the manufacturer’s instructions. In brief, 30–50 mg of flash-frozen tissue per sample was dissociated with a pestle, incubated for 10 min on ice and washed. Dissociated tissue was passed through a nucleus isolation column, and flowthrough nuclei were washed in debris removal buffer and wash and resuspension buffer. Nuclei were quantified with a Nexcelom Bioscience Cellometer. For maximum targeted recovery, 40,000 nuclei per sample were loaded for Gel Bead-In Emulsion generation. Samples were processed by the Chromium Next GEM Single Cell 3′ HT Dual Index v3.1 protocol and sequenced to a depth of 550 million read pairs per sample (around 23,000 read pairs per nucleus) on a NovaSeq 6000 system (Illumina).
Spatial transcriptomics analysis using whole-genome spatial transcriptomics (VisiumHD)
A 7-μm-thick section from an 8 d.p.i. FFPE sample was placed in a histology slide, dried at 42 °C for 3 h, dehydrated overnight, baked at 60 °C for 30 min and deparaffinized following the VisiumHD standard protocol (CG000685). The mouse reagents (VisiumHD, Mouse Transcriptome, 6.5 mm, four reactions, PN-1000676) were used to obtain high-quality H&E images, perform hybridization, RNA removal and probe amplification, carry out analyte transfer to the VisiumHD slide using the Cytassist with the 2.0 software, and generate libraries, which were sequenced following the recommended configuration in a NovaSeq 6000 Illumina instrument. Binary base call files were demultiplexed into FASTQ files using spaceranger mkfastq followed by spaceranger count to generate the spatial representation of gene counts by matrix at 2-mm-resolution and 8-mm-resolution binning. A Cellpose40 cell-segmentation model was fine-tuned to segment nuclei in the high-resolution VisiumHD H&E image. A cell-by-gene matrix was created by summing transcript counts in all 2 mm bins overlapping each cell in the segmentation mask. gimVI was used to jointly embed cells from an 8 d.p.i. Xenium dataset with those from 8 d.p.i. VisiumHD41. The crypt–villus axis and cell types for VisiumHD were imputed by using the three closest Xenium neighbours in the joint latent space. The crypt–villus axis values were calculated as the mean of the neighbours’ values, whereas the cell type was determined by the most frequently occurring type among the neighbours. Further details of the downstream analysis of the VisiumHD dataset are provided at GitHub (https://github.com/Goldrathlab/Spatial-TRM-paper).
Spatial transcriptomics analysis using multiple error-robust fluorescence in situ hybridization
Fresh frozen tissue was sectioned according to standard histology procedures to a thickness of 10 μm. The sections were adhered to the MERSCOPE slides (Vizgen, 20400001) coated with fluorescent beads by storing them in the cryostat at −20 °C for at least 5 min. The samples were fixed in 5 ml of fixation buffer containing 4% paraformaldehyde in 1× PBS that was preheated to 47 °C and incubated for 30 min at 47 °C, according to the MERSCOPE Quick Guide Modified Fixation for Fresh Frozen Samples. The samples were then washed three times with 5 ml PBS, 5 min each time. The samples were permeabilized in 5 ml 70% ethanol at 4 °C in parafilm-sealed dishes overnight and stored in these conditions for up to a month. Samples were then prepared according to Vizgen’s protocols, starting from the cell-boundary protein-staining step. The samples were hybridized with a custom 500-gene panel that included 5 sequential genes, as well as several blank barcodes that do not encode a gene and used for measuring the background signal. To clear the samples of lipids and proteins that interfere with imaging, 5 ml of Clearing Premix (Vizgen, 20300003) was mixed with 100 μl of proteinase K for each sample, and the samples were placed at 47 °C in a humidified incubator overnight (or for a maximum of 24 h) and then moved to 37 °C. The samples were stored in the clearing solution provided with the MERSCOPE kit in the 37 °C incubator before imaging for up to a week. The samples were imaged on the MERSCOPE according to the MERSCOPE Instrument User Guide. Seven 1.5-μm-thick z planes were imaged for each field of view at 60× magnification. Images were decoded to RNA spots with xyz and gene ID using the Merlin software of Vizgen. Cell segmentation was performed using the Cellboundary algorithm, relying on the Cellboundary 2 stain and DAPI nuclear seeds.
Spatial transcriptomics analysis using 10x Xenium
FFPE tissues were sectioned to a thickness of 5 μm onto a Xenium slide, followed by deparaffinization and permeabilization following the 10x user guides CG000578 and CG000580. Probe hybridization, ligation and amplification were done following the 10x user guide CG000582. In brief, probe hybridization occurred at 50 °C overnight with a probe concentration of 10 nM using a custom gene panel designed to detect 350 different mRNAs. After stringent washing to remove unhybridized probes, probes were ligated at 37 °C for 2 h. During this step, a rolling circle amplification primer was also annealed. The circularized probes were then enzymatically amplified (2 h at 37 °C), generating multiple copies of the gene-specific barcode for each RNA binding event. After washing, background fluorescence was quenched chemically. The sections were placed into an imaging cassette to be loaded onto the Xenium Analyzer instrument following the 10x user guide CG000584.
Spatial data processing
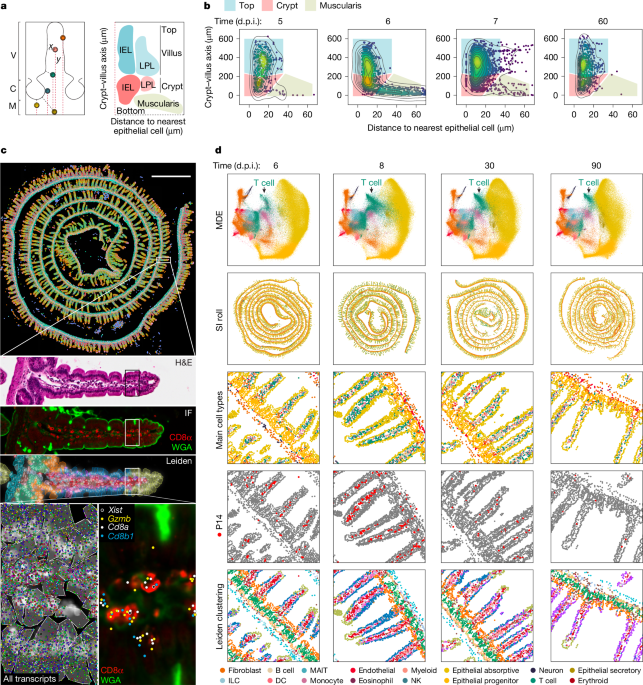
For 10x Xenium spatial transcriptomics data, nuclei were segmented using a fine-tuned Cellpose40 model on maximum-projected DAPI-staining images. Baysor42 was used to predict cell-boundary segmentations using transcript identity and positions, and the prior Cellpose nuclei segmentation or Cellboundary 2 segmentation for 10x Xenium or MERSCOPE, respectively. The parameter prior-segmentation-confidence was set to 0.95 for 10x Xenium and to 0.9 for MERSCOPE, and min-molecules-per-cell was set to the median nucleus transcript count (https://github.com/Goldrathlab/Spatial-TRM-paper#preprocessing). Baysor segmentations containing no nuclei were filtered out, and segmentations containing multiple nuclei were split by assigning transcripts to the nearest nucleus centroid in the segmentation boundary. Cell boundaries are visualized as polygons using the alphashape Python package. All cells with n < 8 nuclear transcripts, n < 20 total transcripts or n > 800 total transcripts were filtered out before downstream processing. To integrate spatial replicates into a joint embedding, scVI43 was used with n_layers of 2 and n_latent of 30. The joint embedding was projected into two-dimensional space using scVI.model.utils.mde. Leiden clustering was performed on the scVI learned embeddings using scanpy.tl.leiden with a resolution of 1, and every Leiden cluster was further subclustered at a resolution of 1.2. Celltypist44 and GeneFormer45 were used for a first-pass cell type assignment, with further manual refinement based on the expression of cell type marker genes to define cell types in a class > type > subtype hierarchy. The Anndata46 format was used for all further processing. To align histology images with Xenium spatial coordinates, we used an OpenCV Oriented FAST and Rotated BRIEF47 object to detect key points in the DAPI channel of both histology and Xenium images. These key points were then matched using an OpenCV DescriptorMatcher, enabling the computation of a homography matrix based on the top matches using cv2.findHomography. Subsequently, histology images across all channels underwent warping using this homography matrix with cv2.warpPerspective. To align H&E images with Xenium spatial coordinates, we trained a pix2pix generative adversarial network48,49 to predict DAPI images from H&E as an intermediate state before finding key points and matching, as previously mentioned. To visualize mouse transcriptional signatures onto human datasets, all (n = 8) mice time course samples were used to find the top 15 differentially expressed genes (Scanpy rank_genes_groups and method = ‘wilcoxon’) between P14 cells gated to the crypt and P14 cells gated to the top of villi. Human homologues of these 15 genes are defined as UCell50 signatures and mapped to human CD8αβ T cells. Human CD8αβ T cells are positioned on IMAPs and coloured by their enrichment of the (left) top mouse signature and (right) crypt mouse signature. All codes to analyse the spatial datasets are available at https://github.com/Goldrathlab/Spatial-TRM-paper.
Histological staining of mouse intestinal tissue after Xenium analysis
After the Xenium run, slides were kept hydrated in PBS-T (0.05% Tween-20 in PBS) at 4 °C. For post-Xenium immunofluorescence staining, PBS-T was removed and samples were blocked using universal blocking reagent CAS-Block and stained with anti-CD8a antibody (Abcam, EPR21769) 1:50 dilution) overnight at 4 °C, followed by three washes with PBS. Anti-rabbit AF594 secondary antibody (Invitrogen, A-11012, 1:200 dilution) in Dako antibody diluent was then added for 1 h at room temperature in the dark, followed by three washes with PBS. The slides were then stained using WGA–FITC followed by DAPI staining and then mounted with a coverslip using Vectashield mounting medium. The slides were dried for 1 h at room temperature in the dark before imaging with an Olympus VS200 Slide Scanner at 20×. The slides were then soaked in PBS at 4 °C overnight to dismount the coverslip and subsequently washed three times in PBS and twice in ddH2O before proceeding with H&E staining. The coverslip was mounted using xylene-based mounting medium (Cytoseal XYL Epredia), and the slides were dried for 1 h and imaged used VS200 Slide Scanner at 20×.
Human samples
Deidentified human FFPE samples from healthy participants were acquired from the San Diego Digestive Diseases Research Center. Slices of 5 mm were obtained using a microtome, deparaffinized and H&E stained according to common histology practices or processed according to the 10x Xenium protocol.
Human participants and ethical statement
The Human Research Protection Programs at the UCSD reviewed and approved the protocol, including a waiver of consent. Submucosal ileal biopsies were obtained from patients who underwent colonoscopies to rule out inflammatory bowel diseases. Ileal biopsies were evaluated by a pathologist and found to be normal without histological inflammation. Samples were deidentified and processed for the study.
Gene panel design for probe-based spatial transcriptomics profiling of mouse SI
The gene panel design made use of Predictive and Robust Gene Selection for Spatial Transcriptomics (PERSIST)19, a deep learning model that uses single-cell RNA sequencing (scRNA-seq) data to learn a binary mask for the identification of a subset of genes that best predict cell type from gene expression (supervised) or for the reconstruction of whole-transcriptome gene expression (unsupervised). For the Xenium mouse 350-gene panel, 79 SI canonical cell type marker genes were compiled from existing literature and Xist, a marker for transferred female P14 CD8 T cells. An additional 158 genes from a Nichenet database21 of ligand–receptor pairs were included. Next, supervised PERSIST was run on an immune-enriched gut scRNA-seq dataset51 with the previously compiled set of genes as prior information, adding an additional 70 genes. Finally, supervised PERSIST was run on a SI scRNA-seq dataset to capture 59 cell type marker genes for 350 total targets. To create the Xenium human 422-gene panel, we created a base set of canonical immune marker genes, ligand–receptor pairs, spatially differentially expressed genes in mouse P14 CD8 T cells, and the 10x Genomics base human colon panel totalling 343 genes. Using this set as prior information for PERSIST, and a reference human immune cell scRNA-seq dataset52, unsupervised PERSIST filled in the remaining 79 genes. To create the 494-gene panel for MERSCOPE, we compiled 18 published bulk RNA-seq datasets profiling different immune populations in different disease settings5,53,54,55,56,57,58,59,60,61,62,63,64,65,66, including the ImmGen RNAseq database (immgen.org), and manually curated metadata attributes for each sample for the following categories: cell.main, cell.type, cell.subtype, cell.state, model and tissue. The annotated integrated dataset compilation was used as input for feature selection with XGBoost67. Top genes in each attribute were incorporated as the panel backbone. Furthermore, gene markers of intestinal cell markers from PanglaoDB and ref. 68, ImmGen CITE-seq protein markers and 159 genes from the ligand–receptor database NicheNet21 were added. A total of 494 final genes passed the quality-check filtering of Vizgen for transcript length and expression levels. The Xenium mouse 480-gene panel was designed using the 350-gene panel but removing genes that were minimally informative based on the time course data and adding genes that were relevant based on manual curation and a prioritization score that evaluates their ability to differentiate clusters in a scRNA-seq dataset containing most cell types of the mouse SI69.
Defining structural axes in spatial transcriptomics datasets of the SI
To calculate the longitudinal axis, a multiline segment was initially labelled across the base of the basal membrane, using labelme70, starting from the outermost section of the roll. For each cell, the nearest neighbour was calculated from the set of all locations on the multiline segment positioned closer to the centre of the SI roll. The relative position of the nearest neighbour along the length of the entire multiline segment was used as the longitudinal position. In datasets with atypical morphology, longitudinal axis values for each cell were predicted using a deep neural network trained on a feature space of transcriptional neighbourhood decomposition latent factors and multiline segments marking the base of the basal membrane, the top of each villus and the middle of each villus. Transcriptional neighbourhood decomposition was performed using Scikit-learn71 non-negative matrix factorization on a matrix of the summed transcript count values for the ten nearest neighbours of each cell, calculated with a SciPy72 K-dimensional tree, to create a transformed data matrix W with 15 latent factors. To calculate the crypt–villus axis, a fine-tuned Cellpose model was used to segment villi on the basis of WGA staining in Xenium samples and cell spatial positions in MERSCOPE samples. The distance between each cell and the base of the basal membrane was calculated as before, and these values were z-score scaled among cells in the same segmented villus. For datasets with poorly defined morphology, crypt–villus axis values for each cell were predicted using a TensorFlow v2.18.0 (https://www.tensorflow.org/) deep neural network trained on a feature space comprising a decomposition of latent factors for epithelial and stromal transcriptional neighbourhoods. Transcriptional neighbourhood decomposition was performed by fitting a non-negative matrix factorization model on a matrix of the summed transcript count values (10, mouse; 30, human) for the nearest epithelial and stromal neighbours of each cell in all datasets to avoid the influence of variability in immune populations at different infection states. Crypt–villus axis predictions for each cell were smoothed over their nearest 150 neighbouring cell predictions in human data. To calculate the epithelial axis, the mean distance from each cell to the five nearest epithelial cells was divided by the mean distance to the five nearest cells of any cell type. The resulting values were z-score scaled and clipped at an upper bound to align epithelial distances between the villus and basal membrane.
Generation of IMAPs and transcriptional IMAPs
The epithelial IMAP axis values were computed through a biexponential transformation applied to the clipped epithelial axis values across all cells. Each cell was positioned on the IMAP according to its corresponding crypt–villus axis and transformed epithelial axis values. The density in the scattered point cloud was visualized using colour-mapped scipy.stats.gaussian_kde values, with density lines overlaid using seaborn.kdeplot for enhanced clarity and interpretation. Gate boundaries were drawn manually to distinguish the muscularis, villus crypt and villus top by observing the IMAP locations of cell types known to localize to each region. Transcriptional IMAPs were coloured by adding an array of gene expression counts as a point weight parameter to the scipy.stats.gaussian_kde function. Similarly, gene signature IMAPs were coloured using the squared UCell signature enrichment scores as the point weight parameter. In human IMAPs, signature and gene expression point weights were squared to overcome bias in CD8αβ+ physical density in IMAP visualization.
Statistical analysis
In Fig. 2e, significance cut-offs are set at absolute Spearman ρ > 0.05, an arbitrary threshold used to highlight differences between axes. The Spearman coefficients for each gene and their corresponding P values are documented in Supplementary Table 3 across n = 87,387 P14 T cells. In Extended Data Fig. 7a, the top four differentially expressed genes per condition across P14 cells (n = 4,135 WT, n = 4,161 TGFβR2 knockout) are calculated using Wilcoxon testing with Benjamini–Hochberg correction in the function scanpy.tl.rank_genes_group. In Extended Data Fig. 7d,e, a non-parametric two-sample Kolmogorov–Smirnov statistic is used to calculate the significance of the difference between P14 cell type proximity distributions in wild-type and TGFβR2 knockout conditions. A cut-off of similarity is arbitrarily positioned at a Kolmogorov–Smirnov statistic of 0.08, corresponding to a corrected P value of approximately 1 × 10−12 to 1 × 10−10 (Kolmogorov–Smirnov P values vary with the number of samples in the compared distributions). Kolmogorov–Smirnov tests are documented in Supplementary Table 8. In Fig. 4d,f and Extended Data Fig. 1h, gene-expression counts were log-normalized, and two-sample, two-sided t-tests were used to test for significant differences in mean gene expression pairwise between perturbation groups at a significance level of 0.05 and Benjamini–Hochberg correction for P values in each pairwise group. A similar approach was used in Extended Data Fig. 8e using raw detected barcode numbers without normalization. For each subplot in Extended Data Fig. 1g, we applied one-way and two-way analyses of variance, with Dunnett’s method for multiple comparisons. In Extended Data Fig. 1j,k, we used a one-way analysis of variance followed by Tukey’s honestly significant (HSD) difference tests to create confidence intervals. In Extended Data Fig. 9g, human gene expression counts were log-normalized before differentially expressed genes were calculated between human CD8αβ T cells gated to different regions using a two-tailed Wald test in the Python package diffxpy. P values were adjusted using a Benjamini–Hochberg correction. Data are mean ± s.e.m. in all the figures.
Cloning and making retrovirus
The LsgC plasmid was generated by using PCR to linearize the backbone of the LsgA plasmid and exclude the Ametrine reporter gene73. NEBuilder HiFi DNA Assembly was then used to insert a HA-tagged mCherry sequence, synthesized by Integrated DNA Technologies (IDT), into the open site. ChopChop was used to design sgRNAs targeting mouse Cd19, Thy1 and Cxcr3 (ref. 74). Forward (5′-CACCN) and reverse (5′-AAACN) primers forming the sgRNAs were synthesized by IDT. Each sgRNA was assigned a 388-bp or 390-bp barcode, containing seven or eight probe hybridization sites, respectively. These sites corresponded to one of the three lowest-expressed genes in SI P14 CD8 T cells from the SI spatial transcriptomics time course: Muc5ac, Neurog3 and Fer1l6 (Supplementary Table 7). Each 40-bp hybridization site was separated by at least 10 bp containing no homology with the mouse transcriptome. Barcodes were ordered from IDT as gBlocks Gene Fragments in tubes and were cloned into the LsgC vectors on the 3′ side of the mCherry using NEBuilder HiFi DNA Assembly. sgRNAs were inserted into their corresponding LsgC-barcode vector by digesting BbsI restriction sites, followed by room-temperature ligation (T4 DNA ligase, NEB) with the annealed forward and reverse sgRNA primers. LsgC barcodes were transformed into DH5α competent cells (Thermo Fisher). The three unique LsgC barcodes were separately transfected into platinum-E (PlatE) cells (Cell Biolabs, no authentication or mycoplasma contamination test) to make retrovirus. One day before the transfections, 2.5 × 105 PlatE cells were plated on 10-cm dishes in PlatE medium (89% DMEM, 9% FBS, 1% HEPES 1 M, 1% penicillin-streptomycin-glutamine (PSG) (100×, Thermo Fisher) and 0.1% 2-mercaptoethanol (BME)). PlatE cells were transfected using a mix containing 10 μg of LsgC-barcode vector, 5 μg of PCL-Eco (Addgene, 12371) and TransIT-LT1 (Mirus). Retrovirus was collected at 48 h and 72 h after transfection and stored at −80 °C until use.
Transductions and spatial transcriptomics with pooled perturbations
One day before transduction, splenic P14 CD8 T cells were isolated from a Cas9–eGFP+ donor mouse through negative enrichment, and plated in T cell medium (TCM) (89% RPMI, 9% FBS, 1% HEPES 1 M, 1% PSG (100×, Thermo Fisher) and 0.1% BME) containing 1:500 anti-CD3e (Fisher Scientific, 50-112-9591) and CD28 (Fisher Scientific, 50-112-9711) on a six-well plate precoated with 1:30 goat anti-hamster IgG (H+L; Thermo Fisher Scientific) in PBS and stored at 37 °C overnight. Furthermore, an untreated six-well plate was coated with 15 μg ml−1 of retronectin (Takara Bio) in PBS and stored in the dark at 4 °C overnight. During transduction, the retronectin was removed and the plates were coated with TCM and incubated at 37 °C for 30 min. After removal, the three treated plates were coated with a corresponding LsgC-barcode retrovirus over two successive 30-min incubations. Activated cells were resuspended in a 1:1,667 IL-2 in TCM mixture and spread equally across the three retronectin-treated plates. Corresponding retroviruses were added to each well, and the plate was centrifuged at 2,000 rpm for 40 min at 37 °C. The sgRNA knockouts were validated by performing flow cytometry on the transduced cells 2 days after transduction using anti-THY1.2 antibody (30-H12, BioLegend, 1:200 dilution) and anti-CXCR3 antibody (CXCR3-173, eBiosciences, 1:200 dilution) with mCherry+ anti-CD8a+ (53-6.7 BioLegend, 1:200 dilution) cells gated as successfully transduced. One day after transduction, mCherry+ GFP+ cells were sorted from each of the three transduced populations and pooled 1:1:1, then 1 × 105 cells were transferred into each recipient mouse. Recipient mice were immediately infected with LCMV and euthanized at 7 d.p.i. for spatial transcriptomics.
Computational analysis of pooled perturbations in spatial transcriptomics
To stringently identify perturbed CD8 T cells in the spatial transcriptomics datasets, we identified all cells for which the sum of raw transcripts for Cd8a, Cd8b1 and Cd3e was greater than or equal to 3, that had at least one barcode detected, that belonged to a CD8 T cell cluster and that had ≤1 Muc2 transcript. Fer1l6, the pseudogene for sgCXCR3, shows low expression in goblet cells, requiring a stringent filtering of Muc2 to minimize the possibility of goblet transcript bleed-over falsely marking a CD8 T cell as perturbed.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.