Mosquito stocks
The Ae. aegypti Liverpool strain (LVP) was provided by O. Akbari (UCSD) and was used as the wild-type strain in this study. The trpA11 (referred to as trpA1â/â and trpA1ECFP in ref. 17), Gr191 (referred to as Gr19â/â and Gr19DsRed in ref. 17) and Orlando (ORL) Ae. aegypti strains were provided by L. Vosshall (Rockefeller). The trpA11 line was outcrossed to the LVP line for five generations and a homozygous line was regenerated. We previously described the op11, op12, op21and op22 strains, their double-mutant versions (op11,op22 and op12,op21) and outcrossing of these lines51. Since this publication, we have adopted a new nomenclature for naming mutant and transgenic lines. The updated and previous allele names, respectively, are: op11 = op1R, op12 = op1G, op21 = op2R, op22 = op2G. The An. stephensi strain was obtained from O. Akbari (UCSD). All mosquitoes for each line were randomly selected for analysis at 1â3 weeks of age. We used females exclusively, because only females display host-seeking behaviour.
Mosquito rearing and maintenance
Mosquitoes were raised in 28â°C chambers with 80% relative humidity under 14âhâ10âh lightâdark cycles. Mosquito larvae were hatched in reverse osmosis water and reared using fish food (TetraMin Tropical Granules, 16122, Tetra). Adult mosquitoes were maintained on a 10% sucrose (w/v) solution. For propagating mosquitoes, females were blood fed on a membrane feeding system (SP6W1-3, HemoTek) containing defibrinated sheep blood (DSB250, HemoStat Laboratories). The rearing and maintenance procedure was approved and monitored by the Institutional Animal Care and Use Committee at UCSB.
Set-up to conduct IR-preference assays
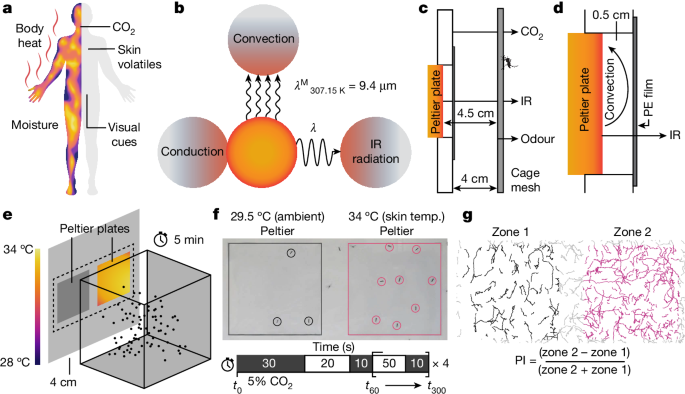
The arena used for behavioural assays was custom fabricated by the UCSB Physics Machine Shop. Five panels of 0.5âinch acrylic (8560K268, McMaster-Carr) were machined as described in Extended Data Fig. 1a. Two 10âcmâÃâ10âcm cutouts were made in one panel with enough tolerance to hold the two Peltier plates (10âcmâÃâ10âcm) securely (TEC plate, model TCP-50, Advanced Thermoelectric). The panels were assembled using stainless steel socket head screws (McMaster-Carr). An LED light bar was mounted to illuminate the wall that contained the Peltier devices (B07CVCF8JF, YEEZEN).
All but one of the interior faces of the arena panels were covered in white PVC adhesive (ConTact, Kittrich) paper to limit unwanted visual stimuli in the mosquito visual field and produce high contrast images (dark mosquito bodies versus light background) for subsequent object tracking. The Peltier plates were also covered with white ConTact paper (emissivity, 0.92). Moreover, the Peltier devices housed in the arena wall were recessed from the interior face of the arena by 0.5âcm to provide an air gap when it covered (Extended Data Fig. 1b). Behind the panel that was left clear, we mounted a webcam (Logitech c920, Logitech) trained on the arena wall that housed the Peltier plates (Extended Data Fig. 1b). Experiments were video recorded using the Logitech Webcam Software (v.2.51).
The arena was designed to accommodate 30âcmâÃâ30âcmâÃâ30âcm mosquito cages (BugDorm-1, DP1000, MegaView Science) placed inside the arena from the top. To improve the imaging quality, we replaced one mesh panel of the cage with a 1/16âinch thick, clear acrylic panel (8560K171, McMaster-Carr) (Extended Data Fig. 1c), and these are referred to as assay cages throughout this study. This modification enabled us to achieve a sharper, higher-contrast image, which improved our tracking ability. The clear acrylic panel was held in place with small machine nuts and bolts (92000A155, 91828A220, McMaster-Carr). The side of the cage facing the Peltier thermal targets was covered with fine polypropylene netting, which is highly transparent to IR.
An automated CO2-release system was constructed using a 12âV Electric Solenoid Air Valve (KL04010, BEDUAN) that was controlled by an Uno R3 Controller Board (EL-CB-001, ELEGOO) and a 12âV Relay Module (HiLetgo). The controller board was programmed with a custom Arduino script that opened and closed the solenoid valve at predetermined intervals. The CO2 was released in the arena through perforated tubing surrounding the Peltier plates (Extended Data Fig. 1d). To measure the CO2 dynamics during a typical experimental regime, we placed a CO2 sensor (CO2 Meter Gas Measurement Specialists, 030-8-0010, K30 FR fast response 10,000âppm CO2 sensor) inside the assay cage on either the left or right-hand side directly centred in each zone. We measured the increase in local CO2 concentration for 5âmin using GasLab software. We found that the absolute CO2 concentrations ranged from 500 to 800âppm when recorded from a CO2 sensor placed inside an assay cage and exposed to the 5âmin CO2 paradigm used in the study. We found no difference in left/right CO2 concentration (Extended Data Fig. 1e). This system allowed for the reproducible release of CO2 at set intervals across experimental replicates. Wiring schematics and code are available on request.
Human odour was applied by wiping a worn glove across the entire front surface of the cage mesh 5â10 times. As the temperature of the surface of both sides of the cage was the same (Extended Data Fig. 2a), increased odour volatility due to a higher temperature on one side is not an issue. In Extended Data Fig. 8a, human odour was applied as described above to only one half of the front cage mesh. To conduct these experiments, no thermal IR source was used (both Peltiers were at the ambient temperature of 29.5â°C).
Behavioural assays for measuring responses to IR
Unless indicated otherwise, 80 female mosquitoes were manually aspirated from the grouped rearing cage and placed in modified experimental cages the day before experimentation, where they were allowed to feed on 10% sucrose ad libitum. The age of the mosquitoes used for the behavioural experiments was 1â3 weeks. Most of the experiments were conducted in the subjective morning (zeitgeber time 1â5), during which the endogenous host-seeking activity is high (Supplementary Fig. 1a). We used larger numbers of mosquitoes for the mock-antenna-dissected and distal-antenna-dissected groups (175 and 300, respectively) to achieve HSIs in the 5â20 range, as these mosquitoes exhibited an overall reduction in host-seeking activity.
For a single given condition, each cage (biological replicate, n) was tested a minimum of three times (technical replicates). If replicates met inclusion criteria, they were averaged and each average was used to calculate the mean response of that cage (n). If a cage did not achieve two suitable replicates, it was not included in this study. For the experiments in Extended Data Fig. 8a, we did not use an HSI criterion of 5 because CO2 + odour elicits a weaker HSI than CO2, odour and IR together.
Testing behaviour using the set-up with a second PE film 2 cm from cage mesh
To provide an additional test to verify that convective warming from the 34â°C Peltier is not reaching the surface of the mosquito cage, we modified the set-up described above (see the âSet-up to conduct IR-preference assaysâ section) and added a second PE film 2âcm from the first PE layer and 2âcm away from the cage mesh where mosquitoes land. The temperature was recorded at the surface of the cage mesh and at the surface of the second PE film over a 5âmin time span using a temperature probe (TSP01-USB Temperature and Humidity Data Logger; Thor Labs). Preference assays were performed with wild-type (LVP) females using this modified set-up in which we exposed one side to IR from a 34â°C source, and both sides were exposed to human odour and 5% CO2.
Testing response to IR using a one-way choice assay
To test whether IR is an effective cue in a one-way choice assay we modified our set-up (see the âSet-up to conduct IR-preference assaysâ section) and used a single Peltier device with a convective barrier similar to the two-way choice experiments. The behavioural assays were performed with wild-type (LVP) female mosquitoes across a range of Peltier temperatures (28â37â°C) in the presence of CO2 and human odour. The HSI was calculated as the average number of female mosquitoes host seeking at the cageâs mesh directly opposite to the single Peltier device throughout the 5âmin experiment.
Set-up for measuring effective distance for detecting IR
To assay the distance over which female mosquitoes are able to detect thermal IR, we first took into consideration that the average surface area of the front of an adult human trunk is ~0.28âm2. This is based on an estimate that the total surface area of an average human is 1.7âm2 (https://www.calculator.net/body-surface-area-calculator.html), that the trunk consists of around 36% of the total surface area52 and that approximately 45% of the trunk faces the mosquito from the front or back. To conduct our experiments, we used an IR source that was 0.22âm2 (45.5âcmâÃâ49âcm), which is a conservative estimate of the area of an IR source available to a mosquito. We covered a 0.44âm2 (91âcmâÃâ49âcm) electric plate made out of stainless steel (Hatco, GRS-36-I) with white ConTact paper (emissivity, 0.92), and then heated the plate to 34â°C. To block IR from half of the plate, we placed a clear acrylic panel 10âcm in front of one half of the plate (Fig. 2i). To prevent convection heat from the 34â°C IR surface reaching the mosquito cage that we used to perform the behavioural assays, we placed a high-IR-transmitting PE film in front of the cage. An acrylic panel was placed as a barrier between the IR and non-IR sides to prevent any bleed-through of IR radiation to the non-IR side. The CO2 was released at the IR-facing surface of the cage through perforated tubing attached to the top of the front panel of the cage (Fig. 2i). Human odour was applied by wiping a worn glove across the entire front surface of the cage mesh 5â10 times.
To conduct the behavioural assays, we transferred 80 female mosquitoes into the assay cages and allowed them to acclimatize for a minimum of 24âh, while they were allowed to feed on 10% sucrose ad libitum. We then placed the cage at different distances from the IR surface and monitored host-seeking behaviour for 5âmin by recording movement after landing of mosquitoes on the mesh facing the IR and non-IR sides in the presence of human odour and 5% (v/v) CO2.
Set-up to assay responses to all three modes of heat transfer from a 34â°C source
To modify our set-up to allow the mosquitoes to be exposed to all three forms of heat transfer (conductive, convective and radiant) from a 34â°C source, we removed the convective barrier (PE) and situated two Peltiers (one at 34â°C, and one at 29.5â°C) flush against the cage mesh, therefore allowing all forms of heat transfer to be present in the assay. To conduct the behavioural assays, we transferred 80 female mosquitoes into the assay cages and allowed them to acclimatize for â¥24âh, during which they were allowed to feed on 10% sucrose ad libitum. We then placed the cage inside the arena and monitored their host-seeking behaviour for 5âmin by recording movement after landing on the cageâs mesh flushed against the Peltier devices in the presence of human odour and 5% (v/v) CO2.
Set-up to assay responses to all three modes of heat transfer from a 50 °C source
To expose the mosquitoes to all three forms of heat from a 50â°C Peltier source, we modified the set-up (see the âSet-up to conduct IR-preference assaysâ section) by removing the convective barrier (PE) and placing the single Peltier device set at 50â°C flush against the cage mesh. This allowed the mosquitoes to be exposed to all three forms of heat transfer: convection, conduction and radiant. The behavioural assays were performed in the presence of CO2 and human odour. Preference assays were performed in the modified set-up described directly above with either intact wild-type (LVP) females, or with female mosquitoes in which the distal ends of the antennae were removed. We tested the aversion of the mosquitoes to 50â°C in a conduction/convection/IR setting (landing on plate) where the plate was 50â°C and the cage surface was exposed to human odour and 5% CO2. The PI of the mosquitoes landing on the 10âÃâ10âcm 50â°C Peltier plate (zone 1) versus landing on the surrounding area of the same size (100âcm2; zone 2) was calculated (PIâ=âHSI of zone 1âââHSI of zone 2/HSI of zone 1â+âzone 2).
Air temperature recordings
A temperature probe (TSP01-USB Temperature and Humidity Data Logger, Thor Labs) was positioned inside the behaviour cage 4âcm away from the wall of the behaviour arena, directly opposing the Peltier plates. With the convective barrier either in place or removed, we recorded air temperatures for 5âmin under various conditions. We measured the air temperature in front of the Peltier set to a range of temperatures (28â37â°C) as well as the control Peltier (turned off, equilibrated to ambient conditions). Moreover, we shielded the temperature probe with aluminium foil to prevent incident IR from heating while still allowing ample air space around the probe to allow for measurements. We therefore calculated the mean temperature value as well as the minimum and maximum recorded values (Extended Data Fig. 2a,b).
Quantifying IR using a pyroelectric detector
To measure IR levels across different Peltier temperatures, and different distances from the Peltier, we used a DLaTGS (deuterated l-alanine doped triglycine sulphate) type pyroelectric IR detector (Bruker, D301) featuring a potassium bromide window with a spectral range spanning 1â25âμm and a sensor surface area of 1âmm2. The signal from the IR source was modulated through an optical chopper at 20âHz (Scitec Instruments, 340CD), and the signal was measured with a lock-in amplifier (Stanford Research Systems, SR530).
To measure the IR signal from the Peltier device at different temperatures (28â°C, 31â°C, 34â°C and 37â°C), we initially constructed a standard plot of black body radiation emitted from black insulation foam at varying temperatures. The total energy emitted by the black body surface was determined using the StefanâBoltzman law. Subsequently, we computed the energy at the sensor by considering the inverse square dependence and assuming uniform angular emission.
The fraction of radiation intercepted by the detector was calculated as the ratio of its area (1âmm2) to the area of the hemisphere with the radius equal to the distance between the IR sensor and the IR source. This gave us the black-body radiation incident on the sensor area from the sample area of the black body. The values obtained for the Peltier device at different temperatures (28, 31, 34 and 37â°C) were then scaled by the ratio of the measured signals in mV (from the lockin amplifier) to the black-body signal. This adjustment enabled us to derive the intensity of the IR signal at different temperatures (28â37â°C).
Similarly, to calculate the IR signal intensity at different distances (8, 10, 15, 20, 25 and 30âcm) from the Peltier at 34â°C, the fraction of radiation intercepted by the detector was calculated as the ratio of its area (1âmm2) to the area of the hemisphere with the radius equal to the different distances between the IR sensor and the IR source. This gave us the black-body radiation incident on the sensor area from the sample area of the black body. The values obtained for the Peltier device at different distances (8â30âcm) were then scaled by the ratio of the measured signals in mV to the black-body signal. This adjustment enabled us to derive the intensity of the IR signal at different distances.
Set-up to assay responses to IR blocking using IR filter window
We used silicon (Si) IR windows (100âmm diameter and 0.5âmm thickness with a small notch on the edge) that have a transmission range spanning 1.2â7âμm, although, even in this range, the Si wafer reduced the IR transmission. The Si wafer effectively blocked most of the IR wavelengths above 7âμm (Soka Technology, P100). As the blocking efficiency depends on the window thickness, we used multiple Si wafers to create a doseâresponse for IR blockage. Variations in thickness (1âmm, 2âmm and 3âmm) were used to effectively block IR from 34â°C IR source. The efficiency of the IR blockage was checked using IR thermography. As the dimensions of the Si wafer were different from the Peltier dimensions, we cut out cardboard to match the dimensions of the Si wafer and placed the cardboard with Si wafer in front of the 34â°C Peltier. The background of the images was normalized by setting the same colour space in the colour distribution settings of FLIR Ignite software. The IR-blocking experiments revealed that the 3âmm thick IR block window substantially blocked the IR from the 34â°C source. To perform the behavioural experiments, the same IR-blocking Si wafers were placed in front of both Peltiers to prevent any visual bias. Preference assays were conducted with wild-type (LVP) females in this modified set-up with different thicknesses of IR block windows, where one Peltier was at 34â°C and other at ambient 29.5â°C. Both sides were exposed to human odour and 5% CO2.
Development of object-tracking MATLAB scripts
We used an automated tracking and scoring program for several reasons. First, automated video analysis substantially increases the throughput of experiments compared with other conventional manual scoring methods. Second, automated scoring reduces the opportunity for scoring bias that could arise during manual counting methods. Third, automated scoring enabled us to selectively study and score those mosquitoes actively host seeking on the cage mesh (Supplementary Video 1) and not those that are stationary.
We initially tried commercially available or open-source tracking programs; however, they were either too cumbersome, time consuming or ineffective in tracking mosquitoes well. We therefore developed a bespoke set of scripts to track and score our experiment recordings using MATLAB (MathWorks). All of the code described in this study is available at the Craig Montell Lab GitHub repository (https://github.com/Craig-Montell-Lab/Chandel_DeBeaubien_2023).
The tracking program generates a thresholded image (black and white) in which the mosquitoes appear as black blobs against a white background (Supplementary Fig. 1b,c). The centroid (centre) coordinates of these blobs are recorded and stored for every video frame of the experiment (99.8% were 5âminâÃâ10âfpsâ=â3,000 total frames). To selectively study host-seeking mosquitoes, we reconstructed the movements of the same mosquito over time. We used a nearest-neighbour function with a maximum-cut-off value to stitch together coordinates in successive frames. This method enabled us to reconstitute the trajectories of individual mosquitoes throughout the 5âmin experiment recording (Supplementary Fig. 1d,e).
For the videos related to the effective distance for IR detection, we used a nearly identical approach to the one described above, only modifying our method for foreground detection. As there were small changes in the background of these videos as we changed the distance of the cage to the IR source, we opted to create a background model for each video rather than use a fixed pixel threshold. To accomplish this, we randomly extracted 100 frames from each video and then calculated the modal pixel value. The resulting image was used as the background model, as it was devoid of all moving objects (mosquitoes). To identify mosquitoes, we took the absolute difference in pixel value between each frame and the background model, thresholding pixel changes >30 arbitrary units. This robustly identified mosquito blobs in the foreground, which were then subjected to identical filtering as described above and below.
We analysed the mosquito movements to identify quantitative features that would enable us to study host-seeking mosquitoes selectively. First, we isolated the position data from mosquitoes that landed on the mesh of the cage, and removed data from mosquitoes that were flying. To do so, we analysed all of the blob areas (pixels) captured throughout a 5âmin experimental recording. By analysing the distribution of body sizes, we found that those of landed mosquitoes fell within a defined range and were almost always larger than those of flying mosquitoes (Supplementary Fig. 1f). In video recordings, flying mosquitoes appeared less opaque than landed ones, causing their size to appear smaller after image thresholding. Thus, to selectively study mosquitoes that had landed, we thresholded the data of body sizes that fell within a defined range (Supplementary Fig. 1f).
Having isolated mosquitoes that landed on the cage mesh, we wanted to then isolate the data from mosquitoes that are actively host seeking. Here we define host seeking as walking along the cage mesh, which is correlated with probing behaviour (Supplementary Video 1). As we cannot directly observe probing from the vantage point of the recording camera, we use walking movement as a proxy for this behaviour. To empirically determine stationary and host-seeking behaviour features, we manually generated two representative datasets of stationary and seeking mosquito movements. These data were curated from actual experimental data. By analysing the distributions of velocities in these data, we determined a threshold value that, when exceeded, represents mosquitoes in motion (Supplementary Fig. 1g). Note that the seemingly paradoxical velocity of stationary mosquitoes results from slight differences in the calculated centroid position of that stationary object over time, called jitter. The remaining data after these steps represent actively host-seeking mosquitoes and were used for analysis in all behaviour experiments described in this study. The overall host seeking is referred to as the HSI, and is calculated as the total number of host-seeking observations during each video (99.8% were for 5âmin), divided by the total number of frames (for example, 3,000 frames for the 5 min videos). In other words, the HSI represents the average number of mosquitoes that are host seeking at any given time. Moreover, for the experiments shown in Fig. 3a, we calculate the IHSI by dividing the total number of host-seeking mosquitoes in one zone at a given timepoint by the total number of experiments (18 experiments, 6 biological replicates, 3 technical replicates each). This metric represents the average number of mosquitoes host seeking in that zone, at that timepoint. As An. stephensi rarely walked around the back of the cage, we opted not to threshold the IR data based on walking speed and, instead, included data from all landing events.
The automatically determined HSI as described above does not merely measure walking but includes a requirement for probing with the proboscis. This is unlike previously used manual counting approaches that cannot discriminate between random landings and mosquitoes that display behaviour associated with blood seeking. We therefore compared the sensitivities of using the HSI with manually quantifying the number of mosquitoes in each zone every 30âs from the videos. We found that manual counting under-represents the preference for the IR zone in comparison to our automated scoring method (Extended Data Fig. 2c). In total, we recorded 1,483 videos (see Data availability section), 1,480 of which were for 5 minutes. The only exceptions were videos IR 1878 (3.60 min) and IR 1882 (4.89 min), corresponding to Fig. 3e, and SI 10 (4.82 min), corresponding to Fig. 3b.
Optimization of scoring parameters
In this study, we use the PI metric to summarize mosquitoesâ biased or unbiased distribution during a given behaviour assay. This metric takes into account all observations of mosquito host seeking. Thus, if too few data points are fed into this metric, random variation significantly influences the experimental outcome. We wanted to determine an informed rationale for the minimum acceptable HSI for experiments to be included or excluded in this study. To investigate how low response rates impact the overall variance in experimental outcomes, we created a model informed by actual experiment parameters. We first analysed experimental data to identify key features of mosquito movements while host seeking. By examining the directionality of movements, we found that seeking mosquitoes walk mostly upwards with no left/right bias (Supplementary Fig. 1h). Using this information and their average velocities, we created a random walk simulation that approximates the movement duration, velocity and directionality of host-seeking mosquitoes (Supplementary Fig. 1i,j).
To model the effect of HSI on experiment variance, we populated a set number of fictive mosquitoes in a two-dimensional environment with the same dimensions as real experiments (720âÃâ1,280 total, with two 466âÃâ456 scoring zones). We simulated mosquito movement using the previously described movement model. The input number of mosquitoes ranged from 1 to 30 and, at each input number, the model was iterated 10,000 times. We then analysed the resulting movements in the same manner as for the real experimental data, recording both the PI and HSI. As the starting position was uniformly random, we would expect the average of simulated PI outcomes to approximate 0. We found that the distribution of outcomes with low HSIs had a very wide distribution and, in extreme cases, ranged from a PI of â0.99 to 0.95 (Extended Data Fig. 2d). These indicate that, at extremely low HSIs, the ability of the data to represent the underlying preference is poor. Furthermore, there was a nonlinear reduction in variance as the HSI increased (Extended Data Fig. 2d). With this information, we determined a minimum HSI of 5 to be required for the inclusion of an experiment in subsequent analyses. We chose this threshold because there is little additional decrease of variance at higher response levels and, technically, this would require increasing the number of mosquitoes per assay to achieve such HSIs. In this study we analysed 522 biological replicates. Each biological replicate included â¥3 technical replicates. 8% of all technical replicates were excluded because they did not meet the HSI threshold (HSIâ=â5). This resulted in 85.2% of all biological replicates being calculated from the mean of â¥3 technical replicates (445 out of 522), and 14.8% being calculated from 2 technical replicates (77 out of 522).
Correlation studies
To determine what behaviour or behaviours were strongly associated with changes in the PI, we analysed 982 individual behaviour experiments. We first investigated whether a shift in PI was correlated with mosquitoes spending longer time on average in the preferred zone. To do this, we calculated the difference in ATT for each zone by dividing the cumulative host-seeking time spent in each zone by the overall number of tracks (bouts) observed in that zone (Extended Data Fig. 5a). The data for each experiment were normalized to the average host-seeking time spent in all zones, providing a metric with a range of â1 to 1. An ATT score of <1 indicates that, in that experiment, individual mosquitoes spent on average more time occupying zone 1 while host seeking compared with zone 2. An ATT score of >1 would, therefore, show the inverse.
The next metric that we analysed was the difference in ATD between each zone (Extended Data Fig. 5b). One explanation for a strong PI is that mosquitoes are more prone to leave one zone versus another, which would be shown in a skewed ATD score. This was derived in a similar manner to the ATT score, wherein we calculated a normalized ATD differential between zones 1 and 2. An ATD score of <1 would suggest that mosquitoes on average walked longer bout lengths in zone 1 as compared to in zone 2, and the inverse is true if ATDâ>â1.
Finally, we wanted to score the DTT between each zone. This would reflect the total number of mosquitoes navigating to that zone and exhibiting host-seeking behaviour (Extended Data Fig. 5d). To do this, we calculated the normalized difference in total number of tracks, ranging from â1 to 1. A DTT score of <1 indicates that, in that given experiment, there was a greater number of overall host-seeking bouts in zone 1, and conversely more in zone 2 when DTTâ>â1.
RTâPCR and qPCR
To detect expression of trpA1 and opsin mRNAs in the antennae using reverse transcription PCR (RTâPCR) and qPCR, we isolated RNA from 200 antennae using TRIzol Reagent (Thermo Fisher Scientific). We prepared cDNA using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific) with oligo(dT) primers. For RTâPCR, we amplified for 32 cycles using Phusion High-Fidelity DNA Polymerase (New England BioLabs) and for qPCR, we amplified for 40 cycles using the LightCycler 480 SYBR Green I Master Mix (Roche). We used RpL17 as the normalization reference. The PCR primers are listed below. The RNAs prepared from the control and mutants were used to produce the PCR products for the indicated genes (trpA1, op1 and op2) and for RpL17. The PCR products for trpA1, op1 and op2 and for RpL17 were loaded in adjacent sets of wells on the same 1% agarose gel. The original scan from two biological replicates is shown in Supplementary Fig. 3. The data shown in Extended Data Fig. 7a are from the experiment presented at the top in Supplementary Fig. 3.
The PCR primers were as follows: Op1-f (5568060), 5â²-AGAAGAGAAATAGAATGGCGGC-3â²; Op1-r (5568060), 5â²-GAACGCTAGGTTGACCACCA-3â²; Op2-f (5567680), 5â²-CTGTCCGGAGGAGAAGACAATG-3â²; Op2-r (5567680), 5â²-GGTCAGGTAGTCAGTTCCGC-3â²; Op3-f (5568061), 5â²-GGCACTCACTCCTGGGATTC-3â²; Op3-r (5568061), 5â²-TCGTGAGCAGATACAGCCTTAATA-3â²; Op4-f (5566757), 5â²-ATCTGACCGTGGTGGATAGA-3â²; Op4-r (5566757), 5â²-GAAGTCCGAGAAGGCTAGATTG-3â²; Op5-f (5566755), 5â²-AACATGAGCGCTTGTGGAAC-3â²; Op5-r (5566755), 5â²-GTAACCGTTTCAACATTATCAAGTG-3â²; Op7-f (5569125), 5â²-CGTGGTCGCTGGGATTATT-3â²; Op7-r (5569125), 5â²-GATGGTGAACAGTGGGATGT-3â²; Op8-f (5572198), 5â²-ACTATCTGGCATTGGTGCTGG-3â²; Op8-r (5572198), 5â²-ATTTGCATGCACAAGCTGGG-3â²; Op9-f (5576882), 5â²-TTGCGACGGTGTTCTTTTGG-3â²; Op9-r (5576882), 5â²-CATTGCGTAAGGATTTTGATGTTGA-3â²; Op10-f (5566350), 5â²-CGCTACCGGGAATGTTTGGTG-3â²; Op10-r (5566350), 5â²-TCTTAGCAAGGATTGCGGGG-3â²; Op12-f (5566410), 5â²-GGCCAACATCAGTTGTTCCG-3â²; Op12-r (5566410), 5â²-TCGGCTATCGATTGGTTCCG-3â²; trpA1-f (5571938), 5â²-TGTTATCAAAGGTCTCAAGGATGA-3â²; trpA1-r (5571938), 5â²-AACAGGATTGGCATCAGTATCA-3â²; RpL17-f (5574866), 5â²-AAGAAGTGGCCATCATTCCA-3â²; RpL17-r (5574866), 5â²-GGTCTCCGGGTCGACTTC-3â²; Op1-RT-f (5568060), 5â²-CAACCTAGCGTTCTCGGATTT-3â²; Op1-RT-r (5568060), 5â²-GGCCCTTCACGATGACATTAT-3â²; Op2-RT-f (5567680), 5â²-CCAACCTGCTAGTGGTCAAT-3â²; Op2-RT-r (5567680), 5â²-GTAGACGAAGATGGCGTAGATG-3â²; trpA1-RT-f (5571938), 5â²-ACTGTAAACCGTCCATCGTTAG-3â²; trpA1-RT-r (5571938), 5â²-TATCTTGAGCGGTGTGGTAATC-3â².
In situ hybridization
We devised a modified RNAscope53 protocol for whole-mount Aedes staining. In brief, Ae. aegypti probes were designed by Advanced Cell Diagnostics (ACD) to target genes Aae-LOC5571938 (2326â3239âbp of XM_021842755.1, trpA1), Aae-AAEL005776 (875â1770âbp of NM_001358471.1, orco), Aae-LOC5567680 (900â1397âbp of XM_001657569.3, op2), Aae-LOC5568060 (2â1378âbp of XM_001651947.3, op1), Aae-AAEL018153 (1202â2119âbp of XM_021844485.1, brp). RNAscope experiments were performed on whole-mount antennae in Eppendorf tubes. Around 10 antennae were dissected into PBS, washed once with PBS and fixed in 4% paraformaldehyde in PBS for 16âh at 4â°C. Each sample was then dehydrated in a series of 50% ethanol in PBS, 75% ethanol in PBS and 100% ethanol. After the last wash, ethanol was removed completely, and the tissues were air dried at room temperature for 30âmin. The tissues were then treated with 3% H2O2 in PBS for 10âmin to inactivate endogenous peroxidase activity. The samples were then incubated in RNAscope Protease III for 30âmin. Probe hybridizations were performed overnight at 40â°C. The next day, the tissues were washed three times in RNAscope wash buffer (ACD, 310091) for 2âmin each. The tissues were then incubated with amplifier solutions (Amp1â3) contained in the RNAscope Multiplex fluorescent V2 assay kit (ACD, 323100) according to the manufacturerâs instructions. The tissues were incubated in Amp1 for 2âh at 40â°C, in Amp2 for 2âh at 40â°C, Amp3 for 1âh at 40â°C and C1 for 2âh at 40â°C. Between each step, the tissues were washed five times for 3âmin each at room temperature. For fluorescence labelling, a working Opal dye solution was made fresh using a 1:500 ratio of Opal dye (Akoya Biosciences) to TSA buffer. We then added 150âµl of the working solution to each tube containing around 10 antennae and incubated the samples at 40â°C for 2âh. The tissues were then washed in a wash buffer and mounted in VECTASHIELD mounting medium (Vector Laboratories). Experiments were repeated three times on control and mutant antennae samples. Images were acquired using the Zeiss LSM 900 confocal microscope and Leica SP8 resonant scanning confocal microscope. Maximum-intensity projections of full z-stacks were generated using ImageJ. Three-dimensional neuron counting in the acquired z stack of the 13th flagellomere was performed using Imaris (v.10.0.1). Each count was analysed manually in Imaris to remove objects that were not neurons and to add missing or overlapping neurons.
Histology
Isolated antennae were primary fixed by placing them in a 0.1âM phosphate buffer (pHâ7.2), 2% glutaraldehyde solution overnight at 4â°C, and then secondary fixed in 0.1âM phosphate buffer (pHâ7.2), 1% osmium tetroxide solution for >2âh. The fixed antennal samples were then dehydrated with serial dilutions of ethanol and acetone, infiltrated with epoxy resin (Electron Microscopy Sciences, 14310), embedded in PE moulding trays and cured in an oven. The cured preparations were sectioned at a thickness of approximately 1âµm using a glass knife on a Reichert-Jung Ultracut microtome, stained with toluidine blue and observed under a light microscope.
EAG recordings
We used 5â10-day-old female mosquitoes to perform EAG recordings. Mosquitoes were immobilized on glass slides by attaching their thoraxes and abdomens to strips of double-sided adhesive tape. The mosquito heads rested on top of a coverslip pre-attached to the slide. Thin strips of double-sided transparent tape were used to secure the proboscis to minimize movement. The antennae used for the recordings were immobilized on coverslips with a thin strip of double-sided transparent tape placed along the middle part of the antennae. Glass electrodes (World Precision Instruments, 1B150F-3) were pulled on a Sutter Instrument P97 puller and filled with BeadleâEphrussi Ringer solution. The electrodes were inserted into drops of electrode cream (Parker Laboratories Cream Electrode Signacreme Ea, 72 BT/CA; 17-05) placed on the compound eye (reference electrode) and near the proximal end of the 13th flagellomere of the antenna (recording electrode).
To deliver the IR stimuli, water from a hot water bath maintained at 37â°C was circulated through an aluminium plate (4âcmâÃâ4âcmâÃâ1âcm) so that the final temperature of the surface was 34â°C. The stimulus was applied by placing the heated plate 3âcm from the antenna using a manipulator, with the broader 4âcm square surface of the plate facing the antenna. This surface was covered with white ConTact paper (emissivity, 0.92). To establish that the source of IR (the 34â°C block) did not change the temperature at the site of the preparation due to conductive heat, we measured the ambient temperature.
To test whether the mosquitoes were responsive to stimuli, we exposed them to a positive control (mouth puff) before exposure to the 34â°C stimuli, and determined whether or not there was a response in the trace. If a mosquito did not elicit an EAG response to the positive control, it was excluded from the analysis. If there was no response to the IR, we performed a positive control after the stimulus. If the response to the mouth puff was negative, we excluded the data. To measure the change in field potential after stimulus application, the EAG signals were amplified using the IDAC-4 amplifier and digitized using the EAGpro software (Ockenfels SYNTECH). The following formula was used to measure the amplitude change following each stimulus: (value of the peak response within 5âs after stimulus)âââ(average field potential for 5âs before the stimulus).
Statistical methods
Data from preliminary experiments were used to determine the typical s.d. in behavioural experiment results (Ïxâ=â0.12). We predetermined an effect size of ±0.2 change in the PI to be of interest, and therefore an n of 6 replicates for each treatment would be sufficiently powered. All behavioural experiment groups (such as genotype, condition) consisted of 6 (nâ=â6) biological cohorts (biological replicates), and repeated measurements (technical replicates) were averaged. For experiments designed with two groups, significant differences in group means were determined using parametric two-tailed Studentâs t-tests. For experiments with >2 treatment groups, differences in group means were analysed using one-way ANOVA followed by a Tukeyâs multiple-comparison test. Significance is indicated by asterisks.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.