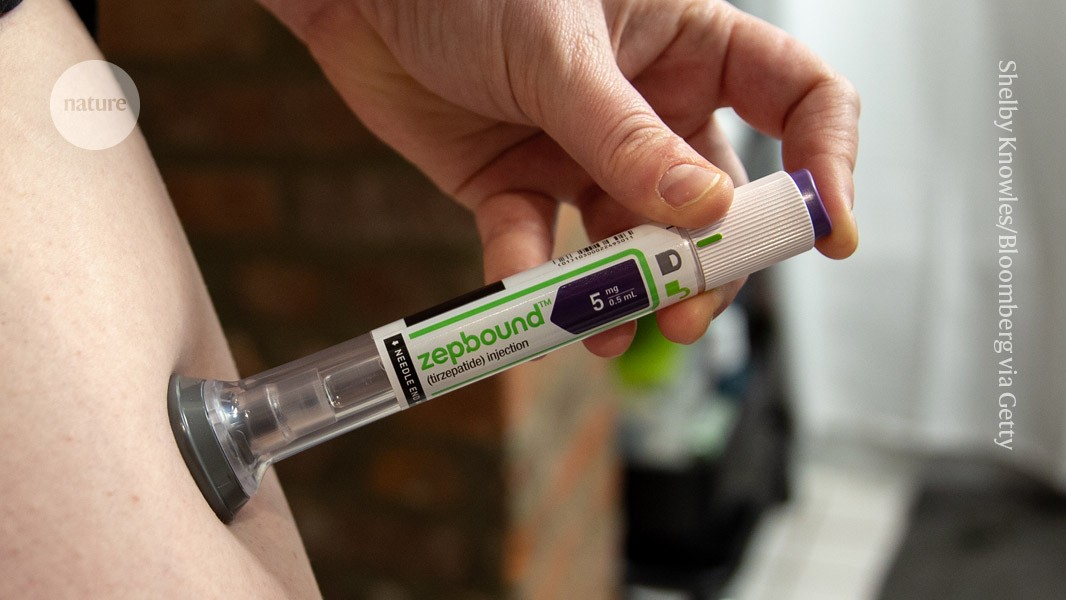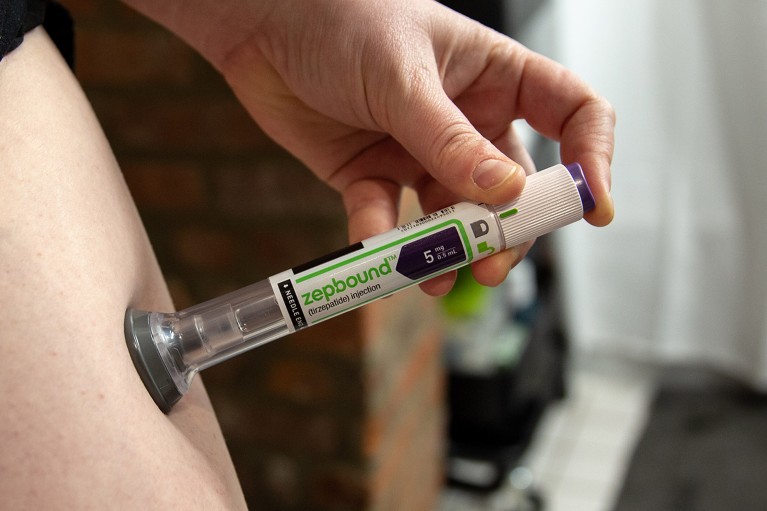
An ongoing trial of Zepbound (tirzepatide) is testing effects on heart disease in people with obesity and diabetes.Credit: Shelby Knowles/Bloomberg via Getty
As appetite for blockbuster weight-loss drugs grows worldwide, scientists are developing the next crop of medications that, they hope, will improve the performance of these drugs and offer benefits beyond weight loss.
“It’s going to be another year where, every week, there’s going to be something really cool,” says Daniel Drucker, an endocrinologist at the University of Toronto in Canada.
New obesity definition sidelines BMI to focus on health
Drug makers are building on the success of medications such as Ozempic and Wegovy, which contain the active ingredient semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist. These drugs mimic the hormone GLP-1, helping to regulate blood-sugar levels and appetite.
But most of these medications are costly, require weekly injections and need to be taken long-term to prevent weight regain. These are some of the issues that companies hope alternative drugs will address.
Nature looks at the weight-loss medicines being tested in 2025 and what they have to offer.
Tirzepatide: cuts heart weight and surrounding fat
Tirzepatide — sold as Mounjaro and Zepbound and given by injection — mimics GLP-1 and another hormone called gastric inhibitory polypeptide (GIP), which is involved in fat metabolism. In trial results published in November, it helped participants to lose up to 20% of their body weight over 72 weeks and reduced the risk of diabetes, compared with a placebo. People taking semaglutide for 68 weeks lose up to 15% of their body weight. Participants who took tirzepatide also had a beneficial reduction in heart weight and in fat surrounding the heart, improved mobility and lower blood pressure and inflammation compared with people on a placebo.
One trial expected to conclude in 2025 is examining tirzepatide’s effects on heart disease in people with obesity and diabetes.
Retatrutide: trial participants report major weight loss
Retatrutide, which has not yet received regulatory approval for weight loss, activates receptors for GLP-1, GIP and glucagon, a hormone that regulates blood sugar levels. Developed by Eli Lilly in Indianapolis, Indiana, it has shown even more promising results than tirzepatide and semaglutide, with trial participants losing an average of 24% of their body weight over 11 months. The drug also helped to reduce blood-sugar levels in people with diabetes, and it’s now being tested in phase III trials that are expected to conclude by 2026.
Anti-obesity drug has life-changing benefits for arthritis
Researchers are excited about combination therapies such as retatrutide, says Beverly Tchang, an endocrinologist at Weill Cornell Medicine in New York City, who notes that obesity’s complexity makes targeting multiple pathways the most promising approach.
Orforglipron: oral drug shows promise
Orforglipron, an oral small-molecule drug candidate also developed by Eli Lilly, showed similar results to GLP-1 injections in rodents, and led to a weight loss of about 10% over 26 weeks in people with obesity, improving blood pressure and levels of circulating fatty molecules. Like Ozempic, orforglipron is a GLP-1 receptor agonist, but whereas Ozempic requires injection, orforglipron comes as a daily pill.
Lilly expects to complete phase III trials in 2025, and orforglipron could receive US regulatory approval in 2026, according to Lilly’s chief executive Dave Ricks.
“The wild card is the oral small molecules,” says Randy Seeley, an obesity specialist at the University of Michigan Medical School in Ann Arbor. Many companies are working on this approach, because pills might be more appealing and cost-effective than injections, but progress has been slower than expected owing to dosing challenges, he says. “If one of them finally breaks through, it has the ability to alter the landscape pretty considerably.”
MariTide: weight-loss maintenance
Biopharmaceutical company Amgen in Thousand Oaks, California, is planning a phase III trial of its drug candidate MariTide, which activates GLP-1 receptors while reducing GIP’s activity. The medication can be taken monthly by injection and resulted in an average weight loss of up to 20% over 52 weeks, according to data released by the company.
Unlike other treatments, MariTide has shown the ability to maintain weight loss for several months after the last dose. When people stop taking semaglutide or tirzepatide, they tend to regain weight quickly, but that doesn’t seem to happen with MariTide, which might encourage more people to have the treatment, says Seeley, who consults for and receives funding from Amgen and other companies developing obesity drugs.