Off a quiet hallway on the top floor of a building at the University of Osaka in Japan, Katsuhiko Hayashi is hatching a revolution.
Human embryo science: can the world’s regulators keep pace?
He is on a decades-long quest to grow eggs and sperm in the laboratory. Hayashi wants to understand the fundamental biology of these reproductive cells. But, if he succeeds, it could forever alter how humans reproduce.
Even for a scientist known for extreme doggedness, it has been a tortuous road. It has taken Hayashi to some strange places: his lab grows fragments of faux ovaries and testes in dishes and has produced mice with two fathers and no mother1.
Every paper he publishes brings e-mails from people clamouring for help with their fertility. “I tell them, ‘This is still experimental’,” Hayashi says. “But sometimes, I can’t respond. There are too many.”
The work that Hayashi and others in the field are doing could offer fresh hope to people struggling with infertility, and to same-sex couples who want children who are genetically related to both partners. But despite the dazzling results researchers have achieved in rodents, that future remains distant. “The technology is super cool,” says Christian Kramme, chief scientific officer at Gameto, a fertility-focused biotechnology company in Austin, Texas. “But fundamentally, I don’t believe there is a single person in the world that should attempt to enact this clinically in the next decade.”
There are plenty of interim goals for researchers to chase, however. Drug companies and regulators hope that a bountiful supply of human gametes — the collective term for egg and sperm cells — could make it easier to test whether drugs and other compounds could reduce fertility or cause mutations that would be passed on to the next generation, says Ina Dobrinski, a reproductive biologist at the University of Calgary in Canada.
And learning how to recapitulate human development in the laboratory could reveal clues about infertility, says Azim Surani, a developmental biologist at the University of Cambridge, UK. “This will be the greatest outcome from these studies,” he says. “Once you understand what causes infertility, you might find ways of overcoming that.”
But as labs move forwards with these experiments, some researchers are raising concerns about potential future uses of the technology. Growing large numbers of gametes in the laboratory could make it easier for parents to select embryos for desirable traits and even facilitate the generation of genetically modified babies.
Although such uses are, by some estimates, at least 15 years away, researchers and some government bodies are calling for regulations to address the potential issues associated with lab-grown eggs and sperm — a process known as in vitro gametogenesis (IVG).
Researchers differ in their estimates of when human IVG will become a reality. Hayashi’s laboratory has produced fertile mouse pups from what he calls egg-like cells grown in the lab2. There’s a chance that human equivalents of these cells could be made in a lab in the next two years, he says.

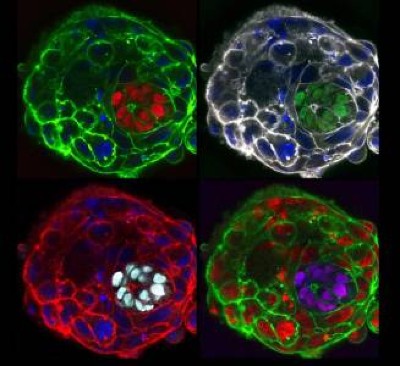
These mice have two biologically male parents. They were generated using eggs made from the males’ skin cells.Credit: Katsuhiko Hayashi, University of Osaka
But that doesn’t mean they’ll be ready for use in people, he adds. Only a small proportion of the mouse embryos generated from his egg-like cells give rise to live pups, and Hayashi speculates that it could take five years or more to produce truly functional human eggs with acceptable success rates.
Scientists will also need to develop and test non-human primate gametes. Ideally, the offspring generated from these eggs and sperm would be tracked for multiple generations, adding years to the timeline. “That’s a critical point,” says Mitinori Saitou, a developmental biologist at Kyoto University in Japan. “My guess is that probably we will have to rely on our next generation of scientists for this.”
A recipe for gametes
For nearly 20 years, researchers have known how to reprogram cells in the human body into induced pluripotent stem (iPS) cells, which can take on nearly any cellular identity. Such cells have been used to make cardiac cells that beat in unison, and nerve cells that conduct electrical signals. But recapitulating the complex and lengthy development of sperm and eggs has proved to be more difficult. “There are so many stages involved,” says Surani. “And each of these is very complex.”
Normally, gametogenesis starts well before birth. Egg development begins in the fetus, in which immature eggs are produced by the millions. Some of these cells then initiate a specialized form of division, called meiosis, in which they lose a set of chromosomes, each cell keeping half the number of an ordinary cell. But this division takes years to complete: the cells pause mid-meiosis and will not resume until years later, after puberty, when they are ovulated. Each immature egg is surrounded by tissue that forms a fluid-filled follicle. These follicles secrete hormones and mature as the egg develops.
Meanwhile, the cells that give rise to sperm also form in the fetus and, after puberty, can produce millions of sperm each day in the winding seminiferous tubules of the testes. Their tubular environment is more complex than the ovary’s follicles, and harder for scientists to recapitulate in the laboratory.
In both cases, the milieu of a developing egg or sperm is crucial. The cells communicate with their surroundings through proteins and other molecules, and receive physical cues from their environment that influence their development.
Immature sperm seem to respond to the flow of fluid around them as it moves through the tubules of the testes, says Hayashi. And the stiffness of tissues in an ovary helps to hold a developing egg in a state of arrest, says Evelyn Telfer, a reproductive biologist at the University of Edinburgh, UK.
The controversial embryo tests that promise a better baby
These signals and scaffolds are difficult to replicate in the lab — especially for human IVG, because it is so hard to obtain tissue to study normal development of the ovaries and testes. Researchers are using techniques that allow them to monitor gene activity and protein expression in individual cells and intact tissue, to pinpoint which molecules and cell types might be important, says Chuva de Sousa Lopes, a developmental biologist at Leiden University Medical Center in the Netherlands.
Some studies incubate human cells with mouse ovarian or testicular cells to encourage them to develop into eggs and sperm3. But this approach cannot not be used to make gametes for use in human reproduction because of possible contamination with animal viruses.
At the University of British Columbia in Vancouver, Canada, urologist Ryan Flannigan and his colleagues are 3D printing testicular tubules from human cells for their IVG experiments4. Other approaches include using microfluidic devices designed to mimic the organs. Dobrinski’s lab grows 3D cell cultures called organoids, which combine several cell types found in the testes.
There are still kinks to work out. Dobrinski’s team, for example, struggles to keep its organoids alive for long enough to grow human gametes. In humans, egg and sperm development takes months3, not counting the years-long wait for puberty, says Kotaro Sasaki, a reproductive biologist at the University of Pennsylvania in Philadelphia. “Many people do not know how difficult it is,” he says.
Gametes in gear
That lengthy time in culture not only slows research, but can also raise the risk of something going wrong, potentially compromising the health of the final product and any offspring, says Eishi Aizawa, a developmental biologist at Harvard University in Cambridge, Massachusetts.
Some groups are trying to make the process more efficient. For example, one team recently found three proteins that each seem able to initiate meiosis in both male and female iPS cells5.
But some features of this meiosis are unusual, and the cells don’t complete their division, says team member Merrick Pierson Smela, chief scientific officer of Ovelle, a biotechnology company in Boston, Massachusetts.
Meiosis is a key stumbling point for IVG, says Paula Cohen, a cell biologist at the Cornell University College of Veterinary Medicine in Ithaca, New York. She and her collaborators have been trying to coax immature human gametes to undergo normal meiosis for about a decade. “It doesn’t work,” she says. Cohen says that many of the teams that have reported success have not fully demonstrated that meiosis is proceeding in their Petri dishes as it would in the body. And proper meiosis is important: errors in the process can lead to gametes with the wrong number of chromosomes.
A different approach, reported last month6, seems to produce functional eggs capable of being fertilized. Researchers replaced the nucleus of an immature egg cell, which has one copy of each chromosome, with the nucleus from a skin cell, which contains two of each. They then triggered a process, which they called mitomeiosis, that discarded one set of chromosomes, mimicking some features of meiosis.
About 9% of these cells led to viable eggs that were fertilized and developed for another six days, generating a precursor to an embryo known as a blastocyst. But all the blastocysts had chromosomal abnormalities, and they did not undergo the genetic shuffling that usually takes place during the development of gametes. This recombination is a source of genetic diversity, and it’s unclear how the lack of it would affect offspring, says Cohen, or how the mitomeiosis process affects subsequent cell divisions. “This would need to be studied intensively.”
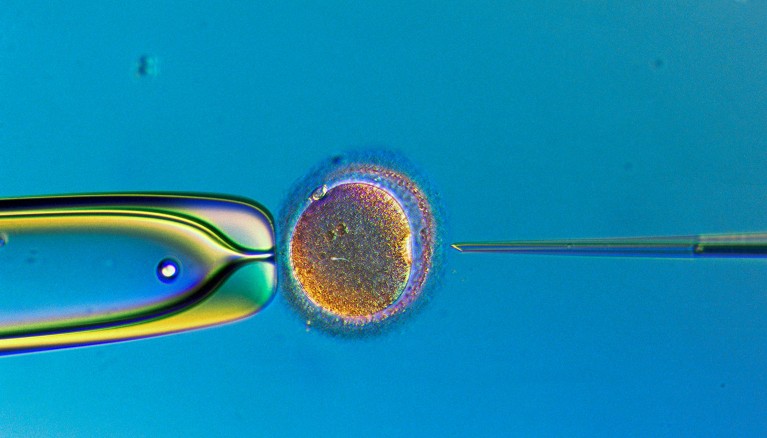
Lab-grown gametes could one day be used for in vitro fertilization.Credit: Lennart Nilsson, TT/Science Photo Library
Another hurdle to achieving human IVG is recreating the complex sequence of DNA alterations that occur at different stages of development. Early in gametogenesis, cells systematically erase certain chemical modifications that can affect gene activity; later, new modifications are added onto certain regions of DNA, some of which are then said to be ‘imprinted’.
This step is crucial: more than a dozen disorders have been linked to improper imprinting, says Surani. “You have to get this right,” he says.