Mouse strains and husbandry
Colony founders of P. maniculatus bairdii (strain BW), P. polionotus subgriseus (strain PO) and P. leucopus (strain LL) were originally obtained from the Peromyscus Genetic Stock Center at the University of South Carolina and then established and maintained at Harvard University. M. musculus (C57BL/6 J strain) were purchased from The Jackson Laboratory. Behaviour, FOS and RNAscope experiments were performed at Harvard and, later, optogenetics, chemogenetics and in vivo recording experiments were performed at Neuro-Electronics Research Flanders (NERF).
Housing at Harvard University: mice were housed on Bed-o’Cobs 1/4-inch bedding (The Andersons) in ventilated standard rodent cages (Allentown) on a 16 h light: 8 h dark cycle at 23 °C with ~20–30% humidity. We provided mice with a red translucent polycarbonate hut, Enviro-Dri nesting material, and a cotton nestlet. All mice were given ad libitum access to irradiated Prolab Isopro RMH 3000 5P74 (LabDiet) and water.
Housing at NERF: mice were housed on Lignocel 3–4 bedding (J. Rettenmaier & Söhne) in ventilated standard rodent cages on a 12 h light:12 h dark cycle at 23 °C with 50% humidity (40–60%). Mice were provided with cotton nesting material. All mice were given ad libitum access to chow diet (ssniff) and water.
After weaning litters at 23 days of age, we kept same sex mice in groups of up to 5 individuals by strain, unless otherwise indicated. Mice of either sex were between two and six months old at the time of experiments.
All experiments were performed as approved by the Institutional Animal Care and Use Committee (IACUC) of Harvard University, and Animal Ethics Committee of KU Leuven.
Animal usage
In total, data were collected from 14 M. musculus and 633 Peromyscus (including P. maniculatus, P. polionotus and P. leucopus) across a series of behavioural, electrophysiological, molecular and optogenetic experiments. For behavioural assays with a sweep–looming stimulus, we tested 14 M. musculus (two trials per mouse), 30 P. maniculatus, 29 P. polionotus and 36 P. leucopus (1 trial per mouse). Repeated looming stimuli with varying contrast were presented to 109 P. maniculatus and 116 P. polionotus (two trials each), while single looming and auditory stimuli, as well as control conditions (for example, hut removal, 2× expansion speed, dimming), were tested in additional cohorts (19–30 mice per group, one trial per mouse). For FOS mapping, repeated looming was used in 21 P. maniculatus and 66 P. polionotus. AAV expression was verified using single-molecule fluorescence in situ hybridization (smFISH) in three mice per species. Neuropixels recordings during evoked behaviour (immersive set-up) were conducted in 6 P. maniculatus and 4 P. polionotus, with 10 and 5 total recordings, respectively; spontaneous activity was recorded in 4 P. maniculatus and 3 P. polionotus using a monitor-based set-up. Optogenetic activation experiments using ChR2 were performed in 7 P. maniculatus and 8 P. polionotus, with controls (no opsin expression) tested in 6 and 5 mice, respectively. Chemogenetic inhibition using hM4D(Gi) and CNO was tested in 13 mice per species; additional groups received saline then CNO (n = 9) or mCherry then CNO (n = 5) per species. Contrast response curves were measured in one mouse per species to complement the main cohorts. Finally, in vitro electrophysiological recordings were conducted in 11 P. maniculatus and 10 P. polionotus. Inclusion and exclusion criteria are described in the corresponding sections below. Unless mentioned there, all trials from all mice were included in the analysis.
Behaviour experiments
Experimental set-up
To assay behavioural response to a visual stimulus, we constructed a rectangular behavioural arena from plexiglass that measured 45 cm (W) × 30 cm (D) × 30 cm (H), adapted from ref. 19. We attached a triangular prism-shaped hut (24 cm (W) × 18 cm (D) × 12 cm (H)) to one corner of arena floor. To reduce reflection, we covered the arena walls and floor with Matte Finish (Krylon). To illuminate the arena, we lined the outside base of the walls with infrared light (IR) LED strips. To record behaviour from below the arena, we made the ground floor of IR-transmissive black plexiglass and used an IR-sensitive camera (Flea3 FL3-FW, monochrome, Point Grey Research) to record at 30 fps. We programmed visual stimuli with Psychtoolbox-3 for Matlab61,62 and displayed them on an LCD monitor from above the arena. Finally, we triggered an LED (invisible to the mouse) simultaneous to the visual stimulus through an Arduino Uno connected to the computer, which we used to synchronize individual frames with the stimulus. We generated sound stimuli with a power amplifier (TB10A, Fosi Audio) connected to a tweeter (Pro-TW120, DS18).
Experimental procedure
Before each behavioural experiment, mice were left undisturbed in their cage for 24 h. We conducted all experiments within the first 4 h of the dark period (Zeitgeber time) and in red light. We habituated mice to the experiment room for 30 min, and then transferred a single individual to the behavioural arena, where it habituated for 10 min. We triggered the stimulus manually when the mouse moved away from the walls towards the centre of the arena and recorded the behaviour of the mouse for 2–3 min before and after the stimulus was triggered. Once testing was complete, we moved the individual to an empty cage and wiped out the arena with 70% ethanol. We then assayed the remaining individuals in the cage following the same protocol.
For the contrast experiment, we randomly assigned a new cohort of mice and exposed each mouse once to a contrast level. Approximately 1 week (range of 5–11 days) after the first exposure, we again randomly assigned the same individuals to a different contrast level and exposed them once. We employed this approach to both minimize habituation from repeated testing and to reduce the number of mice needed for the experiment. For all other experiments (sweep–looming, looming with hut, looming without hut, dimming, auditory), we used new cohorts of naive mice and exposed individuals to the stimulus only once.
To determine which brain region(s) show activity correlated with behavioural response, we collected the brains of mice following their exposure to the overhead stimulus. To this end, we single-housed mice in a new cage the day before the experiment. On the test day, we dark habituated the mice by moving their cage in the test room for 4 h. We then gently transferred mice to the arena, with the hut closed off. After 10 min of habituation, we triggered the stimulus. We recorded the behaviour of mice during the complete trial. We then transferred mice back into the cage and transcardially perfused them after 90 min in the dark (see below).
Stimuli
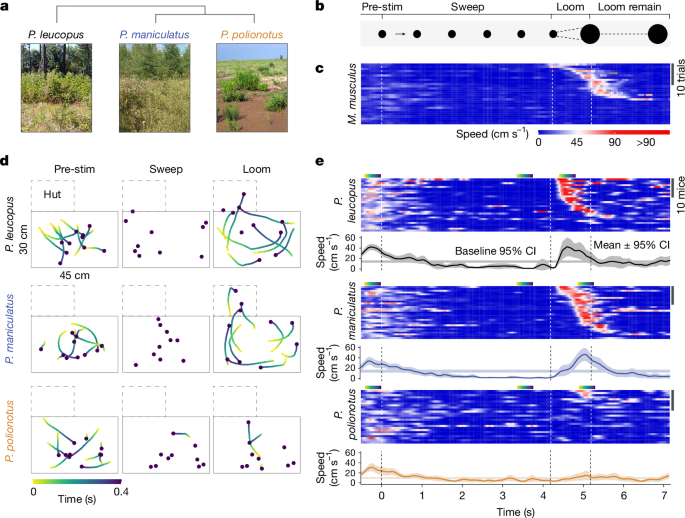
To quantify response to a visual stimulus, we first conducted an assay with a combined sweep–looming stimulus13. The stimulus was a black disc on grey background with a diameter of ~4° visual angle (approximately 2.2 cm) that first appeared in one corner of the computer screen and slowly moved diagonally at a speed of 10° s−1. Once the disc reached the centre of the screen, it rapidly expanded to a diameter of 40° visual angle (approximately 22 cm) at a linear speed of 36° s−1. The disc then remained at full diameter for 2 s before disappearing. We chose these parameters because preliminary experiments revealed that they maximized the difference in behavioural response between the two focal species. For example, a linear expansion speed of 72° s−1 reduced the species-specific responses (Extended Data Fig. 1g).
To measure the behavioural response of mice to different levels of threat, we altered the contrast of the looming disc by changing its intensity against the standard grey background. Intensity is indicated as a positive percentage, converted from the negative Weber fraction27. We used different contrast levels of the looming disc: 32%, 55%, 72%, 86% and 100%, with one additional contrast level for each species within its dynamic range (P. maniculatus: 45%; P. polionotus: 97%). Contrast values were validated with a digital illuminance meter (LX1330B, Dr. Meter). The stimulus comprised 5 repeats of the standard looming stimulus, with a remain time at full diameter of 0.5 s and an inter-stimulus period of 0.5 s (ref. 27).
To test the behavioural response to an aversive auditory stimulus, we exposed mice in the looming arena to an ultrasound frequency upsweep (17–20 kHZ over 1.3 s, repeated 5 times; 80 dB at arena floor), while the visual screen displayed a grey background33,34.
To test the effect of a refuge on behavioural response, we exposed mice to a single looming stimulus (black disc on grey background) in the presence of the hut. As before, the stimulus remained at full diameter for 2 s before disappearing. To test the effect of the absence of a refuge, we closed off the hut and exposed mice to the same single looming stimulus.
To test the behavioural response to a non-moving, innocuous visual stimulus, we used a disc of fixed size (diameter of 40°) that appeared in the centre of the screen, initially matching the grey background but then changing to black over 1 s and remaining black for 2 s before disappearing.
To quantify FOS levels after defensive behaviour, we exposed mice to 125 repeats of the standard looming stimulus, structured into 25 sets of 5 repeats, with a remain time at full diameter of 0.5 s, an inter-stimulus period of 0.5 s within sets, and an inter-set period of 3 s. Control mice were exposed to only the standard grey background.
Analysis
To characterize the behavioural response of an mouse to the stimulus, we used a custom Matlab (v.2015b or newer) code to retrieve centroid coordinates of the mouse and the status of the stimulus from the video recordings. We calculated the speed of each mouse from these coordinates and smoothed the data using a mean filter with a width of five frames.
‘Escaping’ is typically defined as running at speeds above a data-derived threshold and towards a refuge, while the definition of ‘freezing’ varies across studies, but often refers to the absence of movement defined by very low displacement thresholds13,19,55,63. Here, we classified behaviours based on data-derived thresholds, but without additional constraints on movement direction. Specifically, we automatically annotated escape events as a speed ≥55.74 cm s−1, and freezing events as a continuous period of ≤3.28 cm s−1 for at least 0.4 s while the mouse was outside the hut (see Extended Data Fig. 1). We arrived at these definitions by comparing behaviour during exposure to a single looming stimulus to baseline behaviour. For this experiment, we analysed a video segment for each mouse with a duration of 1 s that preceded stimulus exposure by 1–2 min. We selected these video segments such that they matched our criterion for triggering a stimulus (see above; that is, when the mouse moved away from the walls towards the centre of the arena). We recorded escape speed as the maximum speed during the escape event.
For the sweep–looming experiment, trials in which the mouse was in the hut at the onset of the looming stimulus were removed (P. maniculatus, n = 1; P. polionotus, n = 3; P. leucopus, n = 8); we compared only mice that were exposed to the full stimulus.
In the contrast looming experiment, we assumed that mice had definitively detected the stimulus if two independent observers unanimously confirmed a discernible response (that is, interruption or commencement of body movement) during the first looming repeat (Extended Data Fig. 2c,d).
To test for the effect of the presence or absence of a hut, we removed mice that did not show evidence of detecting the stimulus (hut present: P. maniculatus, n = 1; P. polionotus, n = 1; hut absent: P. maniculatus, n = 0; P. polionotus, n = 2).
FOS immunohistochemistry
Immunohistochemistry and imaging
To measure neuronal activity of mice exposed to a looming stimulus, we used the immediate early gene product FOS as a marker of neuronal activity. Following the behaviour experiment described above, we transcardially perfused mice with ice-cold 1× phosphate-buffered saline and then with 4% paraformaldehyde. Brains were dissected out, postfixed for 24 h at 4 °C, cryopreserved in 30% sucrose, and stored at −70 °C until subsequent use. We selected a subset of these mice that represented the species-typical distribution in escape number and speed (Fig. 3b and Extended Data Fig. 3a,b) for FOS immunohistochemistry. To stain for FOS protein, we sectioned brains at 40 μm, blocked tissue for 1 h, and incubated sections for 2 days with rabbit anti-FOS antibody (1:4,000, Synaptic Systems, 226003). We used donkey anti-rabbit Alexa 647 antibody (1:1,000, Invitrogen, A31573) for secondary detection and mounted tissues with DAPI Fluoromount-G (SouthernBiotech, 0100-20). Slides were imaged on an AxioScan.Z1 slide scanner (Zeiss).
Analysis
Following imaging, we exported images to.tif format and arranged sections into anterior–posterior order with the ImageJ plugin TrakEM264. We manually outlined regions of interest (ROIs) with custom Fiji (v.2.1.0 or more recent)65 macros based on landmark structures identified using autofluorescence patterns and DAPI staining. For the dPAG, we included the medial and lateral subdivisions (dm/dlPAG). To segment images, we used the ImageJ plugin StarDist66 with default parameters (model – versatile, normalize image – yes, percentile low – 1, percentile high – 99.8, probability – 0.5, overlap threshold – 0.4), which automatically detects cells using neural network models with star-convex shape priors. For each identified cell in the dataset, we retrieved the area, xy coordinates, and mean intensity. We filtered out large artefacts that were incorrectly identified as cells by removing objects with an area of >180 μm2. Across the ROIs (superior colliculus and PAG) in each section, we then counted cells with mean intensities larger than the mode of the density function of mean intensities as FOS-positive. With this approach, we filtered out cells with the lowest FOS expression, to enrich for cells that were activated during the behavioural experiment, and to remove any batch effects (for example, baseline FOS expression levels across experiments).
Single-molecule fluorescence in situ hybridization
Experimental procedure
To determine whether FOS+ cells were excitatory or inhibitory neurons, we selected, from our previous FOS experiment, three individuals of each species with strong escape responses and high levels of FOS+ cells, as well as three control mice for combined smFISH and immunohistochemistry processing. We obtained six sections (thickness 14 μm) from each mouse, and then used half to detect NeuN (also known as Rbox3), Gad1 and FOS, and the other half to detect NeuN, VGluT2 and FOS. Seven out of the 72 sections did not have reliable staining and were excluded from the dataset. To determine if AAV2+ cells were primarily excitatory or inhibitory, we injected three mice of either species with the viral vector (see ‘Virus injection and fibre implantation’) and then obtained 6 sections (thickness 14 μm) from each mouse and used half to detect Gad1 and YFP, and the other half to detect VGluT2 and YFP.
smFISH protocol
We used the RNAscope Multiplex Fluorescent Reagent Kit v2 with the RNA-Protein Co-Detection Ancillary Kit for co-detection of mRNA and protein. For smFISH, we used custom-made RNAscope probes for Gad1, VGluT2 and NeuN. Probes were based on the coding sequence of each gene, and single-nucleotide polymorphisms were included by alternating between species (P. maniculatus and P. polionotus). For immunohistochemistry, we used rabbit anti-FOS (1:100, Synaptic Systems, 226003) and rabbit anti-GFP (1:100, Thermo Fisher, A-11122) to detect FOS protein and the YFP tag in the viral vector, respectively, and horseradish peroxidase-labelled goat anti-rabbit antibody (1:500, PerkinElmer, NEF812001EA) for secondary detection. We visualized RNA probes and antibodies with Opal 520, Opal 570, and Opal 690 dyes (1:1000, Akoya Biosciences, FP1487001KT, FP1488001KT, FP1497001KT), and counterstained with DAPI. ROIs (mSC, dPAG) were imaged on a LSM 700 laser scanning confocal microscope (Zeiss), with z-stacks of 21 slices spaced at 0.99 μm. We then used QuPath v0.2.3 to quantify the overlap of FISH and immunohistochemistry signals in the maximum projection images.
Analysis
For the FOS–RNAscope experiment, we assigned neuron and transmitter identity to cells by defining section-specific cutoffs as the mode of the density function of the log-transformed distribution of RNA punctae number minus half (for NeuN) or one time (for VGluT2 and Gad1) the standard deviation of the distribution of RNA punctae number. We defined cells as neurons or as excitatory or inhibitory when they had at least three NeuN or VGluT2/Gad1 punctae, respectively, and exceeded the section-specific cutoffs. From this dataset, we then calculated the following three variables: percentage of neurons that co-express a given transmitter, percentage of transmitter-positive neurons that co-express FOS, and enrichment ratio (percentage of transmitter-positive neurons that co-express FOS, divided by percentage of neurons that co-express FOS). For the complete dataset, we then generated a mixed-effects linear model [response ~ (variable + species + stimulus + transmitter + brain region)5 + section ID] using the R package lme {lme4}, and evaluated the model by contrasting stimulus (percentage of transmitter-positive neurons that co-express FOS) or species (percentage of neurons that co-express a given transmitter, enrichment ratio) with emmeans {emmeans} and contrast {emmeans}. We adjusted P values with the Benjamini–Hochberg method.
In vivo Neuropixels probe recordings
Headpost surgery
Mice of each species (2–4 months old) were anaesthetized with isoflurane (Iso-vet; 3% for induction, 1–3% during surgery), placed into a stereotaxic system (Narishige, SR-5N), and dura tear (Novartis, 288/28062–7) was applied to protect their eyes. After removing the hair on the head with depilation creme, we injected lidocaine (Xylocaine 0.5%, 0.007 mg g−1) under the skin above the skull and then incised the scalp along the midline to reveal the skull. A metal headpost was fixed on the skull using dental cement (Superbond C&B, Prestige-dental). The mice received a single injection of buprenorphine (0.2 mg kg−1 intraperitoneal injection) and antibiotics (Emdotrim; 1 ml per 100 ml) in the drinking water for the next 3–5 days.
Experimental procedure
After at least 3 days of recovery, the mice were anaesthetized briefly and a craniotomy above the superior colliculus was performed using a dental drill. Still under anaesthesia, we transported the mice to the recording set-up. For recordings of the same mouse on later days, we briefly anaesthetized the mouse in its cage and transported it to the recording set-up. Mice were fixed with their headpost on a ball floating on air (polystyrene white ball, 20 cm diameter). Two recording set-ups were used (see ‘Recording monitor set-up’ and ‘Recording immersive set-up’).
A Neuropixels probe version 1.0 or phase 3A67 (imec) coated with a fluorescent dye (DiD, DiI or DIO; Thermofisher) was lowered slowly into the right superior colliculus and dPAG. We targeted the centre of the superior colliculus based on anterior–posterior coordinates and the portion next to the midline to detect responses to the upper visual field. We then covered the exposed brain and skull with artificial cerebrospinal fluid (150 mM NaCl, 5 mM potassium, 10 mM d-glucose, 2 mM NaH2PO4, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES adjusted to pH 7.4 with NaOH).
This set-up allowed mice to walk and run on the ball, with tight control over their field of view. Twenty minutes after insertion of the probe, we started the presentation of visual stimuli. Following the recordings, the mice were euthanized, and the probe location was verified by confocal images of the fluorescent dye in 200-µm-thick slices stained with DAPI. We included recordings only in cases in which the probe went through the sSC and dSC as well as the dPAG, and in which we could detect clear light responses in the superior colliculus.
Recording monitor set-up
This set-up was used to characterize visual response properties and to analyse spontaneous escape behaviour. We presented visual stimuli on a 32-inch LCD monitor (Samsung S32E590C, 1,920 × 1,080 pixel resolution, 60 Hz refresh rate, average luminance of 2.6 cd m−2) with the lower part of the monitor placed 35 cm in front of the left eye of the mouse (covering 90° of azimuth and 70° of altitude) and at an angle so that the distance between the eye of the mouse to the left corner, right corner and top of the monitor was comparable. A Neuropixels probe was lowered slowly into the brain and after reaching the target depth, we waited for 20 min for the neural activity to stabilize and for the mouse to settle on the ball. Although head-fixed, due to the possibility to move ‘freely’ on the ball, mice did not require further habituation to the set-up. Once settled, we presented visual stimuli and recorded the neural activity with the Neuropixels probe and the movement of the mouse on the ball with two motion sensors (Tindie, PMW3360). We recorded the 384 electrodes (16 µm lateral spacing, 20 µm vertical spacing) at the tip of the probe, covering 3,840 µm in depth. Signals were recorded at 30 kHz using the Neuropixels headstage (imec), base station (imec), and a Kintex-7 KC705 FPGA (Xilinx). High frequencies (>300 Hz) and low frequencies (<300 Hz) were acquired separately. To select the recording electrodes, adjust gain corrections, observe online recordings, and save data, we used SpikeGLX V20230101-phase30 software (https://billkarsh.github.io/SpikeGLX). We simultaneously recorded the timing of visual stimulation using digital ports of the base station.
Visual stimuli monitor set-up
Visual stimuli were presented on a grey background and were controlled by Octave (GNU Octave) and Psychtoolbox (http://psychtoolbox.org)61,68. Here, we analysed visual responses to 10 repetitions of a black looming disk (from 4° to 50° visual angle in 0.3 s; the disk stayed at full size for 0.5 s before a 3 s grey background) and a dimming disk that stayed at a size of 50° visual angle and changed from grey to black within 0.3 s. All mice were tested under dim daylight conditions (normal screen brightness or 1 log unit darker). For some mice, we conducted additional recordings under moonlight conditions (3–4 log units darker). We used all light conditions to test for a correlation between running speed and neural activity; however, we used only daylight conditions for visual response analysis. To measure contrast response curves, we displayed looming discs of different contrasts in a randomized order with randomized inter-stimulus times of 3–7 s, resulting in 8–12 repetitions of each contrast (5, 15, 25, 35, 45, 60, 75, 90 and 97% Weber contrast).
Recording immersive set-up
This set-up was used to analyse looming-evoked escape. Recordings on the immersive display were performed as in ‘Recording monitor set-up’, with the following differences. Mice were fixed with their headpost in the centre of a panoramic display (50 cm diameter) as described above. Visual stimuli were presented with a projector (DLPDLCR3010EVM-G2 (TI), 1,280 × 720 pixel resolution, 60 Hz refresh rate, average luminance of 5 cd m−2) projecting through a condenser (Canon EF 50 mm F1.4 USM) and a fisheye lens (Peleng 8 mm f3.5 Fisheye Lens M42). The timing of visual stimulation was detected with a photodiode on the fisheye lens. Signals were recorded at 30 kHz using the Neuropixels headstage (imec, HS_1000) connected to a Neuropixels PXIe acquisition module (imec, PXIE_1000) in a PXIe chassis (NI, PXIe 1071). Stimulation timing from the photodiode and ball data acquisition timing were recorded by a PXIe I/O Module (PXIe-6341, sampling rate = 30,488 Hz) in the PXIe chassis. The ball data was recorded using Bonsai V2.6.2 (https://bonsai-rx.org/). An Arduino sent a synchronization signal to both the I/O and Neuropixels modules simultaneously, allowing the alignment of data recorded on the I/O module and the Neuropixels acquisition module.
Visual stimuli immersive set-up
We presented visual stimuli using a custom-made visual stimulation software (OpenGL-based). We presented 20 repetitions of a black looming stimulus on a grey background (97% Weber contrast). Each looming stimulus consists of three presentations of a black disk expanding from 4° to 40° visual angle in 1 s, the black disk remaining at full size for 0.5 s, followed by 0.5 s background (triple loom stimulus). Each set of 3 consecutive looms is separated by a log10 random time interval between 10 s and 3.5 min (((log10(r × 9.9 + 0.1) + 1)/2) × 200 + 10), with r drawn from a uniform distribution in the interval [0,1]. The stimuli were presented at 55° elevation. At the beginning of each experiment, we additionally presented a 4° sweeping white square to estimate the receptive field location of the recorded neurons. The white square was presented at each azimuth ranging from 0° to 80° with 15° intervals on the right side of the mouse. For subsequent stimuli, we selected the azimuth that evoked the strongest response to the sweeping white square.
Spike sorting
We sorted the high-pass filtered neural data using Kilosort269 (https://github.com/MouseLand/Kilosort/releases/tag/v2.0), followed by manual curation in phy2 (https://github.com/cortex-lab/phy). Units were labelled as a real unit based on their waveform shape and auto-correlogram. We used cross-correlograms to identify spikes in different units that belong to the same cell. For subsequent analysis, we used only single unit data. We identified borders between the sSC and dSC as well as the dSC and dPAG using histological sections, spiking activity, and in some cases, clustering of raw spiking activity, similar to ref. 70 (Extended Data Fig. 4). The probe tract was extracted from the tracer labelling together with the known insertion depth. The upper borders of the sSC and dPAG could be clearly identified in the histological sections and the relative electrode numbers were extracted. The border between the sSC and dSC was extracted from the raw spiking activity during looming stimuli and based on previous measurements of sSC thickness using labelling of retinal axons with choleratoxin B (data not shown). Finally, the lower border of the dPAG was based on a conservative estimate from the histological sections, like for the FOS analysis. The placement of the probe close to the midline (that is, upper visual field) minimized the inclusion of lateral PAG neurons.
Contrast response curves
To calculate contrast response curves, we used looming responses of neurons in the sSC. First, we calculated firing rates during each contrast in 100 ms bins and then subtracted background activity before stimulus onset. Then, we averaged firing rates at each contrast and normalized the data by setting peak firing rates at no stimulation (0% contrast) to 0 and the maximum firing rate at any other contrast to 1. We identified responding neurons as cells with: (1) at least 10 significant responses (more than 3× s.d. of mean pre-stimulus activity) at any contrast (out of 90 total stimulations); (2) detectable responses to the highest presented contrasts; and (3) no sudden response drop at intermediate contrasts.
Looming selectivity index
We calculated preferences for looming or dimming stimuli from full-contrast stimuli. We calculated firing rates as the number of spikes in 20 ms bins and extracted average peak firing rate (Pl for looming and Pd for dimming) during multiple repetitions of the stimuli. We defined the looming selectivity index (LSI) as: LSI = (Pl – Pd)/(Pl + Pd).
Locomotion events
In head-fixed mice, escape and freezing bouts were qualitatively similar to freely moving escaping and freezing, i.e. sudden onsets of running bouts or sudden immobility for a short time period. However, there are some differences to the freely moving setting: head-fixed mice tend to move less between stimuli and do not reach the same running speeds. We hence used relative rather than absolute criteria that capture these similarities (sudden onset, large enough change from pre-stimulus behaviour) for extraction of escape and freezing bouts. Specifically, to extract evoked and spontaneous escape and freezing periods, we binned the measured running speed to achieve the same temporal resolution as the neural activity (100-ms bins) and normalized it such that ‘no movement’ is set to 0 and maximum acceleration speed set to 1. Then, we identified time points of onset of escape and freezing. We defined the onset of escape as: an acceleration of >0.2 a.u. within 200 ms after a speed of <0.05 a.u. We defined freezing as: a deceleration (negative speed difference of >0.1 a.u.) from a speed of >0.1 a.u. to a speed of <0.05 a.u. For analysis of spontaneous locomotion, we included only events that were not preceded by a visual stimulus onset in the previous 1 s.
Neural activity during locomotion
We calculated the z-score of neural activity of sorted single units. For data from the monitor set-up, i.e. spontaneous escape events, the z-score was calculated as the firing rate binned in 100 ms bins minus the mean firing rate during seconds −3 to −1 before escape/freezing onset, and divided by the standard deviation across the entire recording. For data from the immersive set-up, i.e. looming-evoked escape events, the mean firing rate and standard deviation was calculated during the 2.5 s before the first looming onset.
Evoked escape analysis
We presented 20 repetitions of a triple loom stimulus (see ‘Visual stimuli immersive set-up’) in an immersive arena to examine how individual neurons encode visual looming stimuli and escape events. Escape responses occurred in a subset of trials. Below we describe three calculations based on this dataset: (1) behavioural selectivity index (BSI); (2) analysis of escape neurons; and (3) linear regression analysis.
Behavioural selectivity index
We calculated two BSIs, one based on correlations and the second based on peak firing rate. For each, estimates of visual responsiveness were based on trials that did not include an escape, and estimates of behaviourally related activity on trials that included a visually triggered escape. To estimate visually related neural activity, we calculated the Spearman correlation between the z-scored neural activity (from non-escape trials) and the disk diameter of the stimulus (scaled from 0 (no disk) to 1 (maximum disk size)). The correlation was calculated over a 10 s window, spanning 2.5 s before stimulus onset to 1.5 s after the final loom. For each neuron, we defined its visual response (Cv) as the average of the three highest Spearman correlation coefficients observed across all trials. Representative examples of single-cell responses for these top three trials are shown in Fig. 4c. To estimate behaviourally related neural activity, we analysed trials in which escape behaviour occurred. We first computed a visual template as the average neural activity of all trials without behaviour and subsequently subtracted this template from each escape trial to obtain the residual neural activity. We then calculated the Spearman correlation between this residual activity and running speed from 2 s before to 2 s after escape onset. The average of these correlation coefficients is the behavioural correlation (Cb). Example single-cell activity during looming trials with an escape event is shown in Fig. 4c (right column). The BSI was then calculated as: BSI = (Cb − Cv)/(Cb + Cv). The data for this version of BSI are shown in Fig. 4d,e.
To evaluate the robustness of our findings, we repeated this analysis using maximum firing rates instead of correlation coefficients. First, we extracted the maximum firing rates during the first loom (FRv) and during escape events (FRb). A behavioural selectivity index based on firing rate (BSIFR) was then calculated as: BSIFR = (FRb − FRv)/(FRb + FRv). The data for this version of BSI are shown in Extended Data Fig. 5e.
Analysis of escape neurons
Putative escape neurons were identified using either correlations or peak firing rates. To identify putative escape neurons using correlations, we compared the behavioural correlation (Cb; as defined above) to a random correlation (Cn) based on a shuffled set of trials. Specifically, Cn was computed as the Spearman correlation between speed traces from escape trials and neural activity during non-escape trials. Neurons were classified as putative escape neurons if they met the criteria Cb > 0 and Cb > Cn. The data are shown in Extended Data Fig. 5c.
To identify putative escape neurons using peak firing rates, we compared each neuron’s peak firing rate to the average response of all neurons. In Mus studies, escape neurons exhibit weak or no visual responses43. Therefore, putative escape neurons were identified as those with a FRb > threshold (9 spikes per s) and FRv < threshold (9 spikes per s). This threshold is the average peak response during escapes of all trials and mice. Results are presented in Extended Data Fig. 5f, with the peri-escape firing rate analysis shown in Extended Data Fig. 5l,p,t.
To evaluate similarities in visual responses between putative escape neurons and other cells, we computed each neuron’s mean response during the first loom presentation. The distributions of these mean responses during the first loom were compared using the Brunner–Munzel test (Fig. 4f).
Linear regression analysis
To assess the relationship between visual and behavioural responses, we fit a linear regression model to predict spike counts from speed and stimulus traces, based on the top three visual trials and all trials where escape behaviour occurred. Neural activity was binned into 500 ms intervals, and the mean spike count per bin was calculated. Notably, different bin sizes (1 s, 1.5 s, or 2 s) did not change the results (all P < 0.0001). Stimulus traces were binarized (0, absence; 1, presence of the visual stimulus) and speed traces and spike counts were standardized using z-scores.
Statistics
Spearman correlations were computed using the corr function in MATLAB and Julia (v.1.11). The two-sample Kolmogorov–Smirnov test was calculated using kstest2 in MATLAB. Effect sizes and confidence intervals were estimated using DABEST. This included bootstrap-based estimations using 5,000 resampled datasets to compute unpaired mean differences (Gardner–Altman estimation). P values were adjusted for multiple comparisons and represent the likelihood of observing the effect size under the null hypothesis. The regression model was implemented using LinearRegression().fit from the scikit-learn package in Python (v.3.6.0 or newer). Model fits were evaluated using explained variance, R². Cells with R² > 0.1 were included in further analysis. To determine the relative contributions of speed vs. visual stimulus, we computed R² ratios: R²_speed_ratio = R²_speed_only/R²_full, and R²_stim_ratio = R²_stim_only/R²_full.
Spontaneous locomotion–activity correlation
To correlate spontaneous movement events and the corresponding neural activity, we calculated the mean neural activity during each event (−2 to +2 s from onset of escape or freezing). We then calculated the correlation coefficient of the speed trace and the average neural activity using ‘corr’ in Matlab across the full 4 s. Only trials with a z-score > 2 during the event were included in Fig. 4. Results did not change for a threshold of 4 and analyses using that threshold are included in Extended Data Fig. 6. We estimated the 95% confidence intervals per species as well as for a Gaussian distribution with mean 0 and the same variance using DABEST71.
Optogenetic activation experiments
Virus injection and fibre implantation
To optogenetically activate dPAG neurons, we injected a viral vector into the dPAG, followed by implantation of an optic fibre. We followed the same procedure as for headpost surgery. Following the craniotomy, we injected 50–100 nl of viral vector (AAV2/CamkII-hChR2(E123T/T159C)-p2A-EYFP-WPRE, UNC vector core, AV5456B or AVV2/CamkII-EYFP for control mice) bilaterally into the dPAG (P. maniculatus, lambda: +0.9 mm, midline: ±0.2 mm, depth: −2.9–3.2 mm; P. polionotus, lambda: +0.8 mm, midline: ±0.2 mm, depth: −2.6–2.9 mm) with a micropipette (Warner Instrument, G100-4) with an open tip of 30 µm attached to a microinjector IM-9B (Narishige). In two mice (one of each species), a modified injection protocol was used in which the virus was injected into the same location but at an angle of 30°. In both cases, we lowered the micropipette to a position 0.1–0.2 mm below the targeted depth for 2 min, and then brought it up to the injection depth. After 1 min, we slowly injected the virus with a hand-wheel. After 5 min, we retracted the micropipette and closed the skin using Vetbond tissue adhesive (3M, 1469). After surgery, we provided antibiotics (Emdotrim, ecuphar, BE-V235523) via drinking water. Either during or approximately 3 weeks after the viral injection, we anaesthetized mice as described above and implanted an optic fibre (200 μm diameter, length 3.5 mm, NA 0.39, Doric Lenses, B280-2304-3.5) above the injection sites (P. maniculatus depth: −2.5–3.1 mm, P. polionotus depth: −2.4–2.6 mm). Due to the large blood vessels at the midline, we first lowered the fibre into the brain lateral adjacent to the central blood vessel and then gently pushed it towards the midline and lowered it to the target depth by alternating steps of moving 100–200 µm down and up until the target depth was reached. We affixed the fibre with dental cement (Sun Medical LTD). After surgery, we injected mice with one dose of buprenorphine (0.2 mg kg−1 intraperitoneal injection) and provided antibiotics (Emdotrim) in their drinking water for 3–5 days. We single-housed mice following surgery and gave them 7–20 days to recover before behavioural testing.
Experimental procedure
To test the effects of optogenetic dPAG activation on behaviour, we briefly anaesthetized mice in their home cage with isoflurane, transferred them to a round arena (diameter: 43 cm) and connected them to a patch cord with a rotatory joint (Thorlabs, RJPFL2). Mice typically woke up within 1–2 min and started exploring the arena. We illuminated the arena with dim red light and video recorded behaviour with a camera (Point Grey Research, FMVU-03MTM-CS or Basler, acA1300-60gmNIR) positioned 53 cm above the centre of the arena. We then used a DPSS laser system (Laserglow Technologies, R471003GX) or a diode laser (Laserglow Technologies, D4B2003FX) to deliver 50 Hz light pulses (10 ms on, 10 ms off) of 473 or 470 nm over 1 s through an optical fibre attached to the optic implant while the mice were freely moving in the arena. We verified laser power at the output of the patch cord without a fibre before and after recording sessions with a power meter (Thorlabs, PM100D with S130C sensor). Laser powers ranged from 0.3 to 24.1 mW.
We began experiments with low laser powers (<1 mW output at the patch cord) and increased laser power in steps of 1–5 mW to find a regime producing consistent behaviour. We then further investigated the effects of differing power levels below and above. Optogenetic triggers were sent manually using an Arduino when the mouse was moving spontaneously through or along the walls of the arena. The session was terminated when the mice stopped cooperating (that is, sat still for a prolonged time or started running erratically); most mice required more than one session to complete the measurements. We recorded videos at 30 Hz using PylonRecorder2 (Basler) or Bonsai72. After optogenetic experiments, mice were anaesthetized with isoflurane and decapitated.
Analysis of virus expression and fibre location
To analyse viral expression and confirm the location of the fibre, we fixed the brains of test and control mice overnight in 4% paraformaldehyde and then sliced them coronally into 200 µm thick slices with a vibratome. We washed the slices three times in PBS/0.5% Triton X-100 and then incubated them with primary chicken anti-GFP (Thermo Fisher, A-10262 1:200) to stain for YFP that was co-expressed with Chr2 or YFP for control mice overnight at 4 °C. After washing with PBS/0.5% Triton X-100, we incubated slices with the secondary antibody (Alexa 488 donkey anti-chicken, Immuno Jackson, 703-545-155, 1:500) and DAPI (Roche, 10236276001) for 2 h at room temperature. After washing with PBS, we mounted slices on coverslips, covered them with mounting medium (Dako, C0563), and imaged them using 10× and 63× objectives on a confocal microscope.
Using this approach, we were able to exclude mice with no or little YFP staining in the dPAG or with incorrect fibre placements (inside the dPAG or >250 µm above the dPAG). For the remaining mice, we then analysed the extent and location of the Chr2 expression and the fibre placement. First, we took confocal images of the 200 µm slice where the fibre was most visible (z-stack, 10× objective). We then loaded raw images into Fiji65 and identified the slice with the brightest YFP staining and with a clear fibre tract. We split the imaged channels (Chr2 and DAPI) and ran the StarDist 2D plugin66 on the Chr2 channel (parameters: model – versatile, normalize image – yes, percentile low – 10, percentile high – 99, probability – 0.2, overlap threshold – 0.4). We measured and saved the area and xy position of each detected, labelled cell with an area <1,000 pixels. We loaded png versions of the maximum intensity projections into Matlab to manually label the extent of the dPAG, the fibre tip, the outline of the ventricle and a control area without detected cells. We quantified the extent of viral infection as the number of detected cells within the dPAG below the fibre tip. Further, we quantified the area of dPAG with viral expression as percentage of the dPAG area with an intensity value above the mean +2× s.d. control area intensity. We analysed the relationship between YFP expression, fibre location, and running behaviour by calculating the correlation (corr in Matlab) between percentage running trials with number of YFP cells, distance between fibre and dPAG surface, and percentage of dPAG area with labelling.
Analysis of optogenetically induced behaviour
We extracted the head position of mice from each video with DeepLabCut73 (v.2.1.9 or newer) and used this to estimate the movement speed of each mouse during optogenetic stimulation. We excluded trials (that is, laser triggers) for which the mouse moved less than 10 cm s−1 on average during the 0.5 s before the laser trigger.
Optogenetic experiments are fundamentally different from the freely moving and head-fixed experiments for two reasons: first, it creates an artificial, highly synchronized activation of a population of neurons that likely is not exactly the same as the activation triggered by a looming stimulus. Second, for practical reasons (that is, the requirement for a light-weight and hence short optic fibre cable), optogenetic experiments were performed in a small arena, limiting the possibilities for speed and behaviour kinetics. To clarify this difference, we used the terms acceleration and deceleration to describe the behaviour observed in this experiment. Acceleration was defined by a forward acceleration during the 1 s period of optogenetic stimulation, as determined by observing both a sharp increase in speed during the stimulus and visual inspection to ensure the movement was forward. Deceleration was defined as a continuous time period (>400 ms) with speeds below 70% of the baseline speed, confirmed by visual inspection. All remaining trials were classified as “other”. We used these classifications to calculate the percentage of acceleration and deceleration trials (Fig. 5g–i).
In addition, we computed a speed index (SI) and adjusted speed index (adjSI) to compare the speed during the main behaviour with a baseline as follows: adjSI = (SpeedBehaviour – SpeedBaseline)/(SpeedBehaviour + SpeedBaseline). For the SI, the ‘baseline’ was calculated as the mean speed in the 0.37 s before laser onset and the ‘behaviour’ was defined as the mean speed during the laser trigger. For the adjusted SI, the main behaviour (behaviour) was centred around the maximum for forward movement trials and minimum for slowing trials. The baseline period (baseline) was defined as the 0.37 s (11 frames) before the behaviour event. In either case, this computation resulted in positive values for acceleration and negative values for trials in which the mouse decelerated. A final parameter used to compare the sham and experimental groups consisted of the steepest slope during the laser trigger. Again, the slope parameter was positive for acceleration and negative for deceleration trials.
To compare triggered and sham mice as well as different laser powers, we performed three statistical tests: an ANOVA test, a two-sample Kolmogorov–Smirnov test (kstest2 in MATLAB) and estimation of effects size and confidence intervals using DABEST71. For the latter, for effect size estimation, unpaired mean difference Gardner–Altman estimation was performed, in which 5,000 bootstrap samples were taken and the mean difference between two groups was calculated together with the confidence interval. The P value reported is the likelihood of observing the effect size if the null hypothesis of no difference is true.
Chemogenetic inhibition experiments
Virus injection
To chemogenetically inactivate dPAG neurons, we injected 50–100 nl of viral vector (AAV2/CamkIIa-hM4D(GI)-mCherry, Addgene 50477- AAV2 or AVV2/CamkIIa-mCherry, Addgene 114469 – AAV2, for control mice) bilaterally into the dPAG. The procedure was the same as in previous surgeries (head-posting, optogenetic experiments). Mice were tested at least five weeks after the viral injection.
Experimental set-up
To assess the effect of chemogenetic manipulation on visually guided behaviour, we used a custom arena (80 cm (L) × 34 cm (D) × 50 cm (H)) with a red-tinted transparent acrylic floor and a display monitor (LG 34UM69G-B, 34 inch diagonal class 21:9, 250 cd m−2 mean luminance, 60 Hz refresh rate) positioned above the arena. Light levels in the arena were kept dim (>10 lux) by adding a logarithmic filter on the screen. In the arena, we included a shelter, with a triangular prism-shape (H: 10 cm, L: 24 cm, W: 11 cm), made from the same material as the platform, and positioned in one corner of the arena. Below the floor, the arena was lined with infrared LED strips. We recorded the behaviour of the mouse from below the arena with a near-IR camera (Basler, acA1300-60g mNIR, 60 fps) with a fixed 6 mm focal lens (Ricoh Lens, FL-CC0614A-2M). Visual stimuli were presented with BonVision V0.11.074. The synchronization of the camera and the presentation of the stimuli were controlled using customized Bonsai workflows.
Experimental procedure
Before each experiment, we briefly anaesthetized mice with isoflurane (<30 s) and intraperitoneally injected CNO (1 mg kg−1) or saline (same corresponding volume). Following the injection, mice were single-housed in a new cage and transferred into the behavioural room to habituate for 30 min. For acclimatization to the arena, mice were allowed to freely explore it for 10 min. Then, three ‘slow’ looming stimuli (36° s−1 expansion, as in Figs. 2 and 3), were presented with an inter-trial interval of log delayed times between 90 to 180 s. We used online background subtraction and tracking of mice using Bonsai and stimuli were automatically triggered when the mouse entered a predefined square shaped ROI, covering ~1/3 of the arena, centred on the side opposite to the shelter. Experiments were interrupted early if the mouse sat in the same location for over 20 min. Between each test, the set-up was cleaned thoroughly with 75% ethanol. Each mouse was tested twice, once with CNO and once with saline. The initial condition was randomized. Between the two sessions, mice were undisturbed for 1–2 weeks.
Stimuli
Each looming stimulus trial consisted of 5 successive linear expansions of a black disc on grey background. The disk expanded from an initial ~4° visual angles to ~40° at a rate of 36° s−1. Once it reached full size, the disc maintained its size for 0.5 s, then disappeared and was followed by 0.5 s delay period before entering the next expansion cycle.
Analysis
We followed the same analysis as for visually included behaviour (Figs. 2 and 3). DeepLabCut73 was used to retrieve centroid coordinates of the mouse. The coordinates of other body parts were used to check for consistency. We calculated the speed of each mouse from these coordinates and smoothed the data using a mean filter with a width of five frames. We defined and automatically annotated escape events as a speed ≥55.74 cm s−1, and freezing events as a continuous speed of ≤3.28 cm s−1 for at least 0.4 s while the mouse was outside the hut.
Ex vivo electrophysiology
Experimental procedure
For whole-cell electrophysiology experiments, coronal slices of dPAG were prepared from 2- to 3-month-old mice. In brief, mice were anaesthetized intraperitoneally with Nembutal (diluted 1:5 in water) and transcardially perfused with ∼25 ml of ice-cold N-methyl-d-glucamine (NMDG)-based slicing solution containing (in mM): 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 0.5 CaCl2, 10 MgSO4, 30 NaHCO3, 5 sodium ascorbate, 3 sodium pyruvate, 2 thiourea, 20 HEPES and 25 d-glucose (pH adjusted to 7.35 with 10 N HCl, gassed with 95% O2/5% CO2). Brains were rapidly extracted and immediately transferred to ice-cold NMDG solution. After removing the rostral part of the brain (Bregma 0.0–0.2 mm) coronal slices (250 µm) were cut from caudal to rostral in ice-cold slicing solution (using a Leica VT1200) and subsequently incubated for ∼6 min in the slicing solution at 34 °C for recovery. Afterwards slices were transferred to holding artificial cerebrospinal fluid, containing (in mM): 126 NaCl, 3 KCl, 1 NaH2PO4, 1 CaCl2, 6 MgSO4, 26 NaHCO3 and 10 d-glucose (gassed with 95% O2/5% CO2). Slices remained at room temperature in holding solution for ~1 h before experiments.
During recordings, brain slices were continuously perfused (32–34 °C) in a submerged chamber (Warner Instruments) at a rate of 3–4 ml min−1 with (in mM): 127 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 1 MgCl2, 2 CaCl2, 25 glucose at pH 7.4 with 5% CO2/95% O2. Whole-cell recordings of dPAG neurons were done using borosilicate glass recording pipettes (resistance 3.5–5 MΩ, Sutter P-1000), using a double EPC-10 amplifier under control of Patchmaster v2 x 32 software (HEKA Elektronik). The following internal medium was used (in mM): 135 potassium gluconate, 4 KCl, 10 HEPES, 4 magnesium ATP, 0.3 sodium GTP, 10 sodium phosphocreatine, 3 biocytin (pH 7.3). Intrinsic properties were recorded in current clamp at 20 Hz and low-pass filtered at 3 kHz when stored. Single action potentials were recorded at 50 Hz and low-pass filtered at 10 kHz. Junction potential was calculated to be approximately 12 mV, data shown are not compensated. For determining neuronal excitability, current injections (−40 pA to +360 pA steps, 10 pA steps) were performed from a holding membrane potential of −70 mV. Rheobase was determined using ramp current injections. For single AP recordings we used conventional bridge balancing and pipette capacitance compensation combined with minimal stimulation intensity to improve separation between AP and stimulation. The series resistance was compensated to 75–80%. Data were analysed using Fitmaster (HEKA Elektronik), spontaneous input was analysed using the Mini Analysis program (Synaptosoft).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.