Preparation of DOC for co-precipitation experiments
Modern-marine analogue DOC
Performing our co-precipitation experiments with modern marine DOC would require the collection of unreasonable water volumes (several thousand litres at typical surface ocean DOC concentrations). We, therefore, instead used large-batch microcosm cultures containing a mixture of marine primary producers and heterotrophic bacteria to generate modern-marine analogue DOC (termed M-DOC). Two diatom strains, Phaeodactylum tricornutum CCMP2561 and Thalassiosira pseudonana CCMP3367 (Bigelow NCMA), were allowed to grow for 4 days in 2 × 300 ml of L1 medium (Bigelow NCMA) placed in an AlgaeTron growth chamber. These cultures were then mixed in a 1:1 ratio, diluted into 2 × 20 l carboys filled with L1 medium and further grown at 14 °C with a light–dark cycle of 12–12 h. The cultures were stirred with a magnet bar (300 rpm) and sparged with filter-sterilized laboratory air for the entire duration. Five days post inoculation, 1 ml of a mixture of 96 uncharacterized isolated marine bacterial strains (provided by the Cordero Laboratory, MIT) was added to each culture. The cultures were grown for an additional 17 days (for a total of 26 days), reaching late stationary phase. The biomass was harvested by centrifugation at 7,000 RCF (relative centrifugal force) for 10 min at 14 °C (Eppendorf, CR30NX) and stored at −20 °C until further use. The total biomass yield was about 2 mg ml−1 (wet weight). The biomass was then thawed, diluted into 18 M Ω cm−1 double-distilled water (Milli-Q; termed DDW) and sonicated using a sonicator probe (Hielscher Ultrasonics) to lyse cells and release dissolved compounds. The lysed biomass was then centrifuged at 7,000 RCF for 10 min at 14 °C and filtered through a 0.2-μm polyether sulfone filter (PES; MilliporeSigma). The filtered solution was evaporated using a rotary evaporator at 40 °C under vacuum to yield dry DOC. Finally, the dried DOC was stored in an anoxic chamber until further use.
Cyanobacterial DOC
Anoxically dried Arthrospira platensis and Arthrospira maxima powders (BioSamara GmbH, Nu3) were processed in an agate ball mill to obtain particle sizes smaller than 20 μm and were subsequently sieved and combined. The powders were then mixed with DDW at a concentration of 1 g l−1 to generate a large-batch cyanobacteria DOC solution (termed C-DOC). This solution was shaken for 48 h on a bottle shaker at 100 rpm and was then centrifuged, filtered, dried and stored exactly as described for modern-marine analogue DOC, above. The product composition and purity were assured by the manufacturers; no supplementary validation was undertaken.
Fulvic acid
Similar to cyanobacterial DOC, commercially purchased (Mark Nature) fulvic acid powder was processed in an agate ball mill to obtain particle sizes smaller than 20 μm and was subsequently sieved. The powder purity was confirmed by Fourier transform infrared spectroscopy (FTIR). The powder was then mixed with DDW at a concentration of 1 g l−1 to generate a large-batch fulvic acid DOC solution (termed FA). This solution was shaken for 48 h on a bottle shaker at 100 rpm and was then centrifuged, filtered, dried and stored exactly as described for modern-marine analogue DOC, above.
Characterization of DOC for co-precipitation experiments
We used absorbance and fluorescence spectroscopy to understand the molecular compositions of DOC involved in our co-precipitation experiments. Fluorescence excitation-emission matrices (EEMs) were acquired using a Horiba Scientific Aqualog spectrometer (Horiba) with a 1-cm cuvette at room temperature. The EEMs spanned excitation wavelengths from 250 nm to 500 nm at intervals of 5 nm and emission wavelengths from 300 nm to 600 nm at intervals of 2 nm. The integration times ranged from 0.5 s to 5 s. To refine resulting EEMs, three corrections were implemented following standard practice: (1) a lamp intensity correction60; (2) adjustments for inner filter effects61; and (3) normalization to Raman units (RU), defined as the measure of Raman scattering intensity in the sample62.
Fluorescent dissolved organic matter (FDOM) content in RU was determined by summing the maximum intensities of fluorescent peaks, including peak A (Ex: 250 nm, Em: 450 nm), peak B (Ex: 270 nm, Em: 304 nm), peak C (Ex: 350 nm, Em: 450 nm), peak M (Ex: 320 nm, Em: 411 nm) and peak T (Ex: 290 nm, Em: 349 nm) (refs. 63,64). Relative contributions of each peak were defined as the ratio of the intensity of a given peak to the total FDOM content. Several absorbance and fluorescence metrics were also identified and used here. First, the fluorescence index (FI) was derived from the emission intensities at 470 nm and 520 nm on excitation at 370 nm (refs. 65,66). Second, the specific UV absorbance at 254 nm (SUVA254) was calculated as the absorbance at 254 nm divided by the total DOC concentration in solution67. Third, spectral slopes were ascertained by fitting absorption data to the equation
$${a}_{\lambda }={a}_{{\lambda }_{{\rm{ref}}}}{{\rm{e}}}^{-S(\lambda -{\lambda }_{{\rm{ref}}})},$$
(1)
where aλ is the absorption at wavelength λ, λref is a reference wavelength and S is the spectral slope; slopes were determined over two wavelength ranges: 275–295 nm (S275−295) and 350–400 nm (S350−400) (ref. 40). Finally, slope ratios were calculated as SR = S275−295/S350−400 (ref. 40).
Iron (oxyhydr)oxide-DOC co-precipitation experiments
General steps relevant to all syntheses
An iron (oxyhydr)oxide synthesis protocol was developed to understand the loading responses of co-precipitated Fe-OC to (1) crystalline iron (oxyhydr)oxide mineralogy (that is, goethite compared with haematite) and synthesis pH; (2) timing of DOC introduction (that is, before or after precursor synthesis); and (3) DOC molecular composition. Specific synthesis protocols are detailed below, and additional synthesis tests (for example, as a function of temperature, duration, dissolved silica concentration, Fe(III) source, absolute Fe(III) and DOC concentrations) are described in the Supplementary Discussion. Broadly, all syntheses involved the addition of Fe(III)-bearing salts [Fe(NO3)3 ⋅ 9H2O or FeCl3 ⋅ 6H2O] to an (anaerobic when necessary) parent solution, followed by pH adjustment to form the poorly crystalline precursor ferrihydrite. To generate crystalline phases, ferrihydrite was then rinsed, pH was adjusted and solutions were allowed to ripen to goethite or haematite depending on the recipe.
In the protocol descriptions below, we designate the timing of DOC addition with ‘+DOC; x’, where x = ‘pre’ for pre-ferrihydrite DOC addition or x = ‘post’ for post-ferrihydrite DOC addition. Furthermore, to understand whether co-precipitated Fe-OC responds to absolute DOC concentrations or to DOC/Fe(III) ratios, we performed two sets of experiments in which we independently adjusted initial parent-solution DOC or dissolved iron concentrations (while holding the other constant) for all synthesis protocols. Importantly, reagent masses appearing below pertain to the standard concentrations for each type of synthesis. These masses were adjusted accordingly to achieve the desired DOC/Fe(III) ratios. A list of all concentrations used for each experiment is reported in the Supplementary Data.
Goethite syntheses
Acidic goethite synthesis
Goethite was formed in an acidic solution using a modified version of the approach developed in ref. 68. First, 101.071 g of reagent-grade Fe(NO3)3 ⋅ 9H2O (Sigma-Aldrich) was dissolved into 250 ml of trace-metal grade 1 M HCl (Sigma-Aldrich) in DDW to form a base solution, which was diluted to a total volume of 500 ml by adding 250 ml of DDW (+DOC; pre). Then, 250 ml of 2 M reagent-grade NaOH (Sigma-Aldrich) was added while maintaining vigorous stirring to increase the pH to about 1.5 and facilitate the formation of a stable brown sol of ferrihydrite. The bottles were then sealed, and ferrihydrite (+DOC; post) was allowed to ripen to goethite, which would typically commence after about 50 days following the procedure described in ref. 68 (that is, at room temperature). To expedite this transformation, we transferred the reaction mixture to an oven held at 50 °C and allowed it to age for 5 days. After ageing, goethite products were centrifuged, rinsed at least three times with DDW to remove excess –OH, dried at 60 °C and stored until further analysis.
Circumneutral goethite synthesis
First, 2-line ferrihydrite was prepared by dissolving 8.0 g of Fe(NO3)3 ⋅ 9H2O in 100 ml of DDW (+DOC; pre) followed by adjusting the solution pH to 7 by adding 1 M NaOH (ref. 42). The solution was vigorously stirred to ensure complete precipitation. Fresh ferrihydrite precipitates underwent a series of rinsing and washing cycles using deaerated DDW (that is, purged with 99.99% pure N2; PanGas). Ferrihydrite was then purged with N2 gas for 2 h to remove any O2 present in solution. The deaerated suspension was transferred to an anaerobic glovebox (O2 < 1ppm; Coy Laboratory Products), where ferrihydrite was aliquoted into glass bottles. Reagent-grade Fe(NH4)2(SO4)2 ⋅ 6H2O (Sigma-Aldrich) was dissolved in a deaerated solution and titrated to pH 7 using deaerated 0.5 M NaOH. This solution (+DOC; post) was then introduced into glass bottles containing ferrihydrite to achieve a final ratio of Fe(II)/Fetotal ≈ 0.19. Glass bottles were then vigorously stirred, sealed with butyl rubber stoppers, crimped, quickly brought to their designated ageing temperature of 20 °C, and allowed to age to goethite for 10–120 days. After ageing, goethite products were centrifuged, rinsed at least three times with DDW to remove excess OH, dried at 60 °C, and stored until further analysis. We were not able to stabilize synthesis pH using organic buffers (see ref. 42), as this would add additional organic carbon to the experimental system. Rather, we targeted lower goethite yields to maintain a pH range of 5–7 throughout the experiments.
Alkaline goethite synthesis
Goethite was formed in alkaline solution using a modified version of the approach developed in ref. 69. First, 13.515 g of reagent-grade FeCl3 ⋅ 6H2O (Sigma-Aldrich) was dissolved in 50 ml of DDW (+DOC; pre). Then, 90 ml of reagent-grade 5M KOH (Sigma-Aldrich) was added while maintaining vigorous stirring to increase pH to about 11 and facilitate the formation of red-brown two-line ferrihydrite. This solution was quickly diluted with DDW to reach a final volume of 1 l (+DOC; post). The bottles were then sealed, and ferrihydrite was allowed to ripen to goethite in an oven held at 70 °C for 7–10 days. After ageing, the goethite products were centrifuged, rinsed at least three times with DDW to remove excess –OH, dried at 60 °C and stored until further analysis. Highly alkaline conditions required the use of Teflon flasks to avoid Si dissolution that may occur in glass vessels.
Haematite synthesis
Acidic haematite synthesis
Haematite was formed in acidic conditions using a modified version of the approach described in ref. 45. First, 5.405 g of FeCl3 ⋅ 6H2O was dissolved in 1 l of 1 mM HCl in DDW (+DOC; pre). The bottles were then sealed, and the solution was allowed to react (+DOC; post) in an oven held at 90 °C for 14 days to form haematite. After ageing, haematite products were centrifuged, rinsed at least three times with DDW, dried at 60 °C and stored until further analysis.
Circumneutral haematite synthesis
Haematite was formed in circumneutral conditions using a modified version of the approach described in ref. 42, which is itself modified from that in ref. 45. First, solutions of DDW, NaOH and reagent-grade NaHCO3 (Sigma-Aldrich) were prepared and deaerated (that is, purged with 99.99% pure N2) for 2 h to remove any dissolved O2. The solutions were then sealed and transferred to an anaerobic glovebox (O2 < 1 ppm) and were individually pre-heated to the final ageing temperature to ensure consistency in initial reaction conditions. Within the glovebox, 13.40 g FeCl3 ⋅ 6H2O was added to 252 ml of DDW (+DOC; pre), then 150 ml of 1M NaOH followed by 34 ml of 1M NaHCO3 were added to the solution while maintaining vigorous stirring to prevent aggregate formation. The bottles were then sealed (+DOC; post), rigorously shaken and allowed to react in an oven held at either 50 °C or 70 °C for 5–30 days to form haematite (at lower temperatures, it is not possible to obtain mono-mineral haematite under laboratory timescales and circumneutral pH). After ageing, the haematite products were centrifuged, rinsed at least three times with DDW, dried at 60 °C and stored until further analysis. Several iron salt concentrations and compositions were tested before choosing the exact protocol described here (Supplementary Data).
Characterization of precipitated and natural iron oxides
To assess the properties and compositional attributes of both precipitated and natural iron oxides, we used a multifaceted characterization approach as detailed below.
Powder X-ray diffraction
X-ray diffractograms were obtained using an Empyrean 3 diffractometer equipped with a Cu-Kα (1.541,84 Å) X-ray source and a PIXcel3D detector. Patterns for laboratory-precipitated haematite and goethite were acquired by step scanning from 10° to 50° 2θ in 0.01° increments at a scan rate of 0.35° min−1. For natural samples, the scanning range was from 10° to 80° 2θ in 0.02° increments at a scan rate of 0.95° min−1. The obtained X-ray diffractograms were automatically matched against the International Center for Diffraction Data database and the Crystallography Open Database.
Scanning electron microscopy and energy-dispersive X-ray spectroscopy
Scanning electron microscopy imaging and energy-dispersive X-ray spectroscopy elemental mapping were conducted using a Zeiss Sigma scanning electron microscope equipped with an QUANTAX energy-dispersive X-ray spectroscopy (Brucker). The measurements were performed at an operating voltage of 20 kV, using an aperture of 60 μm in analytical high current and vacuum mode.
Section preparation
Round, thick, polished sections were prepared by immersing samples in epoxy resin poured into a 1″ round plastic mould. To prevent sample displacement during immersion, the samples were adhered with a double-sided tape. The samples were allowed to dry for 24 h, after which the sections were polished to expose the maximum submerged sample portion and to achieve the desired smoothness (usually ≤1 μm). Thin sections were prepared by first adhering a sample to a glass slide using epoxy. The adhered samples were then ground to a thickness of about 30 μm while ensuring uniformity across the section. After grinding, each section was polished (usually to ≤1 μm) to remove any surface scratches and enhance clarity.
Optical microscopy
Analysis under plane-polarized light was carried out using a Zeiss Axio Scope A1 microscope. Thick and thin sections were observed at varying magnifications to identify the mineralogical phases and their spatial distributions, photographed and documented (see Supplementary Discussion section ‘Geologic descriptions of sampled formations’).
Raman microspectroscopy
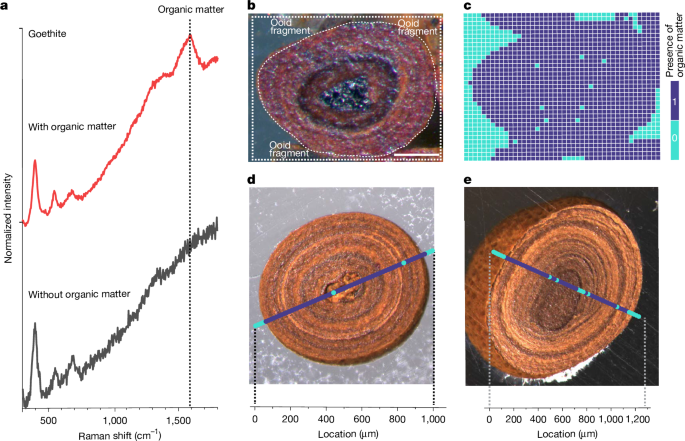
We used Raman microspectroscopy (LabRAM HR Evolution, Horiba Scientific) to investigate the spatial distribution of organic matter in iron ooids. This system integrates a commercial light microscope (BxFM, Olympus) with an optical box for Raman functionality, key components of which include a diffraction grating (300 lines mm−1), a detector (back-illuminated deep-depleted CCD) and a laser (532 nm, 10 mW). The microscope is equipped with objectives and a motorised XYZ-stage for microspectroscopic Raman interrogation of regions of interest within a given sample.
Ethanol pre-sonicated whole ooids (verified by light microscopy) were physically fixed within a cylindrical epoxy mould as described above, thus ensuring a flat surface and enabling measurement of Fe-OC spatial distribution both within and on ooid surfaces. Importantly, sections were polished using carbon-free aluminium oxide powder to prevent carbon contamination. To synchronize visual images of regions of interest with their Raman spectra, light microscopy images were acquired before either one-dimensional raster line scanning or two-dimensional raster area mapping using Raman microspectroscopy (Fig. 1). Considering that sample surfaces contain some roughness (that is, ≤1 μm polishing roughness), a low magnification objective was used (either 10× or 50×; MPlanN or LMPlanFLN, respectively, Olympus). This provides a relatively large depth of focus and thus compensates for Raman signal variation due to surface roughness. Theoretical spatial resolution was calculated to be 1.22λ/NA = (1.22 × 532)/0.25 = 2.6 μm. Therefore, a step size of 8–15 μm was used for raster line scanning and area mapping. For each point measurement, the laser exposure time was 5 s, and the spectral window ranged from 50 cm−1 to 3,100 cm−1.
The presence or absence of Fe-OC in ooid samples was determined using a Raman peak at 1,590 cm−1 (that is, the G-band)70,71 (Fig. 1a). Because haematite exhibits a strong peak at 1,300 cm−1 (that is, near the G-band), the placement of this Fe-OC peak makes the identification of Fe-OC in haematite feasible only if its concentration—and thus signal intensity—is strong72. In an attempt to circumvent this issue, we used other laser wavelengths for haematite ooids (that is, 660 nm and 785 nm), but these spectra did not provide better sensitivity. Owing to this high background from the 1,300 cm−1 haematite peak, the inability to identify Fe-OC cannot be reliably used as evidence for an absence of Fe-OC in haematite ooids. Goethite spectra, in contrast, do not exhibit this behaviour. We, therefore, focus primarily on goethite mineralogy for all Raman line scans and maps.
Raman spectra were processed using software built in-house. A spectral window of 300–1,800 cm−1, which covers Raman signals of goethite and organic matter, was chosen for data processing and analysis. Resulting spectra underwent smoothing (that is, de-noising) using a Savitzky–Golay filter (polynomial order 3 and window size 5) and baseline subtraction using a first- or third-order polynomial algorithm. The presence of Fe-OC was determined by calculating the ratio I1,450–1,700/I1,200–1,450, where I represents integrated intensity within the defined spectral window. These spectral windows were chosen here to represent the range containing the diagnostic Fe-OC peak (that is, 1,450–1,700 cm−1) and that of a background peak that is insensitive to the presence or absence of Fe-OC (that is, 1,200–1,450 cm−1). Using this ratio, a threshold value was selected based on visual inspection of the raw data to convert the calculated values to a binary format, indicating the presence or absence of organic matter (1 or 0, respectively). The threshold values are sensitive to sample-specific characteristics (for example, surface roughness) and are thus recalculated for each line scan or map (for example, Panarea Island threshold = 0.600; Aseri Fm threshold = 0.715; Sillaoru Fm threshold = 0.975; Fig. 1). Although variable and based on visual data inspection, these thresholds are chosen to be conservative (that is, equal to or greater than the maximum measured intensity ratio of line scan or map pixels that are known to be free of Fe-OC, for example of epoxy resin or silicate/carbonate cements).
X-ray fluorescence
To ascertain the elemental composition of extracted goethite and haematite ooids, the samples were ground to powder form and subsequently analysed using a Niton XL5 Plus XRF (Thermo Scientific). Each powdered sample was analysed three times, and the powder was mixed in the measuring cup between runs to ensure signals were representative.
Extraction and analysis of ooid-bound DNA
Microbial community compositions of DNA contained in modern ooids from Panarea Island, Italy44, were determined to assess the potential impacts of iron-oxidizing biofilms. Specifically, ooids were hand-picked and split into two groups that were either subjected to (1) sonication in pure ethanol using a sonicator probe to remove surface DNA contamination (that is, analysis of internal DNA only) or (2) no cleaning procedure (that is, analysis of surface-bound and internal DNA). Both groups were then ground within a laminar flow hood (that is, to prevent biological contamination) to expose internal DNA and maximize extraction efficiency. DNA extractions were then performed on both fractions (3 g per extraction, triplicate extractions per group) using a phenol-chloroform-based approach73. Quantification using a Qubit 3.0 fluorometer and the dsDNA HS Assay (Thermo Fisher Scientific) showed that sonicated ooids yielded DNA concentrations of 0.171 ng μl−1, 0.238 ng μl−1 and 0.211 ng μl−1 (n = 3 extractions), whereas non-sonicated ooids yielded higher concentrations of 0.442 ng μl−1, 0.457 ng μl−1 and 0.441 ng μl−1 (n = 3 extractions); this supports the hypothesis that sonication effectively removes surface-bound DNA.
All extracts were then purified with AMPure XP beads (Beckman Coulter). To perform amplicon sequencing of the microbial 16S rRNA gene, sequencing libraries were prepared following a two-step PCR approach. In the first step, bacterial 16S PCRs were performed using Illumina-adaptor primer pair 515F (GTGYCAGCMGCCGCGGTAA) and 926R (CCGYCAATTYMTTTRAGTTT) and KAPA HiFi HotStart ReadyMix PCR kit (Roche Molecular Systems), targeting the V4–V5 hypervariable regions of the 16S rRNA gene (Supplementary Tables 4 and 5). Products of the first-step PCR were then submitted to Microsynth AG (Balgach, Switzerland) for preparation of Illumina Nextera barcoded second-step PCR libraries and sequencing on Illumina NovaSeq (2 × 250 cycles) (Illumina).
Unfortunately, the quantity and quality of most extracts were insufficient for reliable sequencing results, leading to only one successful analysis of a single non-sonicated extract and no successful analyses of any sonicated extracts. The results are thus interpreted to reflect a combination of surface-bound and internal DNA. For this successful extract, 115,236 reads were obtained after sequencing. Adaptor sequences were removed using cutadapt v.3.4 (ref. 74). Sequence analysis was then performed in R v.4.3.1 using DADA2 (v.1.30) (ref. 75) for quality filtering and amplicon sequence variant (ASV) assignment according to published protocols. As low microbial biomass samples are prone to being contaminated by trace amounts of DNA contaminants from extraction reagents, many taxa (for example, Comamonadaceae, Sporichthyaceae, Sphingomonadaceae and Enterobacteriaceae) were removed based on what was detected in the negative control sample (containing extraction reagents only). Taxonomy was assigned using the SILVA reference database v.138.1 using IDTAXA (threshold = 60) (ref. 76) and DECIPHER v.2.3 (ref. 77).
Removal of ferrihydrite-bound and adsorbed OC
After each synthesis, the products were divided into three fractions and subjected to a washing procedure to step-wise remove OC bound to poorly crystalline phases and OC adsorbed to crystalline mineral surfaces. These fractions were treated as follows: First, one fraction was rinsed three times with DDW, dried at 60 °C and stored until further analysis (termed raw). Second, one fraction was treated with a 1 M HCl solution at room temperature for about 1 h to remove residual untransformed ferrihydrite or other poorly crystalline phases (termed −Fh/PC) (ref. 45). This fraction was then washed, dried and stored as described for the raw fraction. Finally, one fraction was treated with 1 M HCl solution as described for −Fh/PC and was subsequently treated with a 1 M MgCl2 solution at room temperature for about 1 h to chelate and remove any loosely bound (adsorbed) Fe-OC complexes on the crystalline mineral surfaces (termed −Fh/PC− ads. OC) (ref. 78). Each fraction was assessed by XRD to ensure that each step does not influence crystalline iron oxide mineralogy.
Preparation and extraction of geologic iron ooids
Bulk ooidal ironstone samples were initially subjected to gentle crushing using a hydraulic press. On fragmentation, individual ooids were selected from the debris on an aluminium foil using tweezers. For more efficient separation, a strong neodymium hand magnet was used to enhance the ooid yield. Following this, a light microscope inspection ensured the inclusion of only intact ooids (that is, broken ooids were not considered).
Whole ooids subsequently underwent a washing cycle involving three DDW rinses. They were then exposed to a 1 M trace-grade HCl (Sigma-Aldrich) solution for 1 h at ambient conditions to eliminate any poorly crystalline phases and potential carbonates, both inherent to the rock matrix and within the ooids themselves. After acid treatment, ooids were immersed in a trace-metal grade 1 M MgCl2 solution (Sigma-Aldrich) for 1 h to chelate and remove any loosely bound Fe-OC complexes. The ooids were then rinsed three times with DDW and air-dried in an oven at 60 °C and pulverized using an agate mortar and pestle until a fine powder was obtained. The preliminary tests verified that the resulting powder granularity was finer than 20 μm. Finally, the powdered samples were resubjected to the HCl and MgCl2 treatments described above to ensure that the final products are free of carbonates, poorly crystalline phases and adsorbed OC (that is, −Fh/PC− ads. OC).
Analytical methods for measuring Fe-OC content and isotope compositions
Organic carbon contents, reported as wt% OC, and isotope compositions, reported as δ13C in ‰ relative to Vienna Pee Dee Belemnite (VPDB), of iron (oxyhydr)oxides were quantified using a Thermo Scientific Flash Elemental Analyser coupled to a Thermo Scientific Delta V Isotope Ratio Mass Spectrometer (EA-IRMS). Samples were combusted in the presence of O2 in an oxidation column at 1,020 °C. Combustion gases passed through a reduction column at 650 °C, in which the N2 and CO2 gases produced were separated chromatographically and transferred to the IRMS by an open split for online isotope measurements.
For calibration, approximately 30–60 mg of each protocol-treated iron (oxyhydr)oxide sample was aliquoted into triplicate tin capsules. These were crimped and loaded into a helium-flushed autosampler. Both wt% OC and δ13C values were calibrated using in-house standards, including Atropina (δ13C = −21.4‰, 70.56% carbon; Thermo Fisher), Nicotinamid (δ13C = −42.2‰, 59% carbon; Thermo Fisher), Pepton (δ13C = −15.6‰, 44% carbon; Sigma-Aldrich) and Bodenstand 5 (0.141% carbon; HEKAtech). These standards were analysed between about every 10 samples. Furthermore, δ13C was calibrated using the international standards NBS22 (δ13C = −30.03‰) and IAEA CH-6 (δ13C = −10.46‰). Uncertainty was determined as the standard deviation (±1σ) of triplicate measurements; system reproducibility is typically better than 0.2‰ for δ13C.
To estimate ooid formation timescales, actively forming modern iron ooids were additionally analysed for 14C activities—reported as Fm relative to 95% of the 14C activity of the NBS Oxalic Acid I standard in 1950 and corrected to a δ13C value of −25‰ VPDB79. Specifically, 14C activities were measured using an Elementar PyroCube EA connected to an IonPlus mini radiocarbon dating system accelerator mass spectrometer (EA-AMS) operated using a gas ion source80,81. Similar to EA-IRMS analysis, about 30–60 mg of each iron (oxyhydr)oxide sample was aliquoted into tin capsules, which were crimped and loaded into a helium-flushed autosampler. Oxalic acid II NIST SRM 4990C standard was measured for fractionation correction and standard normalization. The background of the EA was assessed with phthalic anhydride (PHA, Sigma, PN-320064-500g, LN-MKBH1376V), which was weighed into the same capsules used for the samples and therefore included the respective possible contamination. Uncertainty was determined as the standard deviation (±1σ) of propagated blank correction and analytical error from 14C counting statistics. Fm values were additionally converted to uncalibrated 14C ages as
$$\genfrac{}{}{0ex}{}{14}{}{{\rm{C}}}_{\text{age}}=-8033\times ln(\text{Fm}),$$
(2)
reported in units of 14C yr bp (ref. 82).
Data compilation
To compare our Fe-OC δ13C record to other OC records, we compiled literature δ13C values for (1) kerogen, (2) crude oil and (3) carbonate-associated OC. All compiled data, including literature references for each data point, are reported in the Supplementary Data. Compilation details are described below:
Kerogen
Kerogen OC δ13C values over the period 0–2,500 Ma were compiled from refs. 83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111. This time range was chosen as it broadly encompasses our Fe-OC δ13C record as well as the Great Oxygenation Event (GOE), which would be expected to affect carbon cycle and thus OC δ13C values. All literature data that are explicitly stated to be kerogen and contain a reported depositional age were included in our compilation. Some datasets exhibit a positive correlation between measured OC content and δ13C value, indicating incomplete carbonate removal during analysis. For these datasets, we only include the δ13C value from the sample with the lowest OC content. Moreover, we specifically focused on samples characterized by conditions below the greenschist facies. This criterion was applied to exclude any potential isotopic alterations that might occur under higher-grade metamorphic influences.
Crude oil
Crude oil δ13C values over the period about 0–600 Ma (that is, the oldest reliable age reported in the literature) were taken from the compilation of ref. 112 and supplemented with data from refs. 90,98,113,114,115,116,117,118,119,120,121,122,123,124. Unlike for kerogen, more care must be taken when reporting crude oil ages because depositional age can differ from host-rock age due to oil migration. We, therefore, considered only literature data that explicitly reported oil depositional age, and we omitted those data points that reported only host-rock age (that is, with unknown oil depositional age). No further filtering was implemented.
Carbonate-associated OC
Finally, carbonate-associated OC δ13C values over the period 0–2,500 Ma were taken from the filtered compilation of ref. 56. The authors of ref. 56 excluded sediments from non-marine, authigenic and heavily metamorphosed settings in their filtering procedure. We adopted this filtered dataset without modification or addition.
Computational methods
All model calculations (see Supplementary Discussion section ‘Estimating DOC concentrations and δ13C values from iron ooid Fe-OC’) were written in MATLAB and carried out on the ETH Euler scientific computing cluster (https://ethz.ch/de/news-und-veranstaltungen/eth-news/news/2014/05/euler-mehr-power-fuer-die-forschung.html).