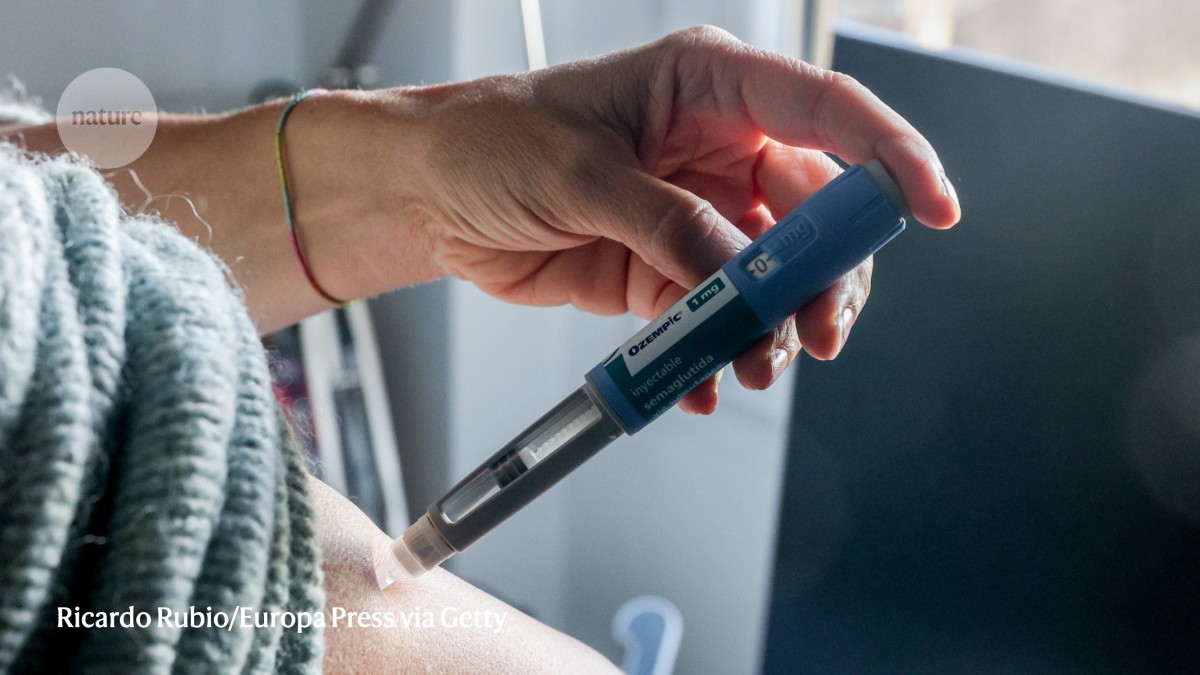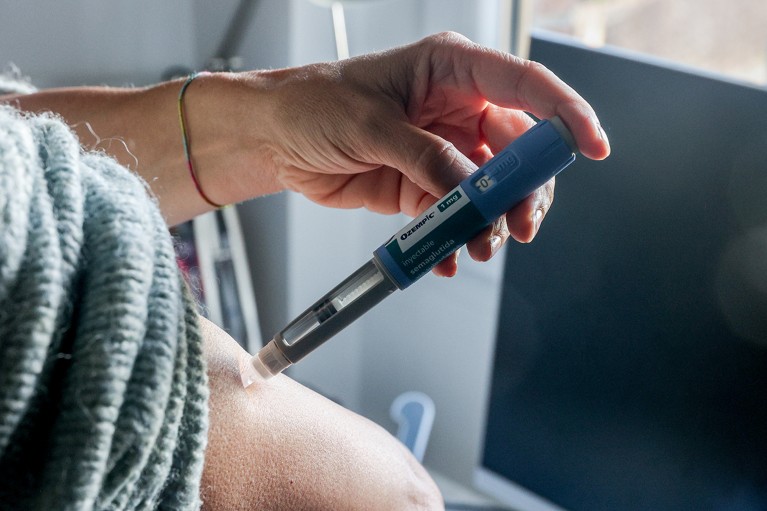
First established as a treatment for diabetes, semaglutide’s popularity as a weight-loss drug has opened the door to new therapies.Credit: Ricardo Rubio/Europa Press via Getty
What do tennis star Serena Williams, television personality Oprah Winfrey and actors Kathy Bates and Whoopi Goldberg have in common? They are some of the many celebrities who have spoken publicly about using GLP-1 receptor agonists to lose weight.
Glucagon-like peptide-1 (GLP-1) receptor agonists — which bind to GLP-1 receptors that are found on the surface of a wide range of cells in the human body — have made their way into popular culture as a byword for weight loss. The drugs even received a mention by comedian Jimmy Kimmel when he hosted the 2023 Academy Awards.
But the treatment is no joke. Endocrinologist John Wilding, at Aintree University Hospital in Liverpool, UK, has spent much of his career seeing people with type 2 diabetes struggle with their weight, fight to maintain diets and exercise regimes, and wrestle with nagging ‘food noise’ — intrusive thoughts about food even when people aren’t hungry. For him, the impact of this new class of drugs is “astounding”.
One of his patients was first diagnosed with type 2 diabetes when she was 17 years old. By her early twenties, the disease had progressed to the point where she was taking three diabetes medications and was facing the prospect of insulin therapy, which can cause weight gain.
Instead, Wilding decided to prescribe her a GLP-1 receptor agonist. The treatment brought the patient’s previously high blood-glucose levels down to the normal range and she lost around one-quarter of her body weight; about 30 kilograms. “It’s totally transformed her life,” says Wilding, who is also a clinical researcher at the University of Liverpool and has been involved in research on GLP-1 receptor agonists. “There’s lots of stories like that, also in older patients, where people have had responses that are really quite remarkable in terms of how much weight they’ve lost and how it’s changed their quality of life.”
The drugs’ popularity has extended well beyond the realm of type 2 diabetes. Between 2019 and 2023, use of GLP-1 receptor agonists by individuals in the United States with obesity surged by around 700%1, as popular brand names found their footing in the market — most notably Ozempic and Wegovy, which are forms of the GLP-1 receptor agonist semaglutide.
“This era of drug development for obesity has the potential to be a landmark in the history of medicine,” says endocrinologist Timothy Garvey at the University of Alabama at Birmingham. “I think it’s on par with the discovery of insulin, the discovery of penicillin, the polio vaccine.”
The first chapter
To trace the origins of semaglutide, one must start with the Gila monster (Heloderma suspectum): a venomous orange and black lizard that is native to the arid deserts of Mexico and the US southwest.
In 1984, after learning of the reptile’s ability to regulate its metabolism and blood-sugar levels even after long periods without food, a group of researchers from the US National Institutes of Health isolated substances from its venom that they thought might play a part. One peptide, which became known as exendin-4, triggered the pancreas to produce and release insulin — a hormone that stimulates cells to take up glucose from the bloodstream after a meal and store it for energy.

Even during fasting periods, the Gila monster (Heloderma suspectum) is able to regulate its metabolism and blood-sugar levels with the help of the peptide exendin-4.Credit: Danita Delimont/Shutterstock
Exendin-4 bore a close resemblance to a human hormone called GLP-1, which also stimulates the production of insulin in response to rising glucose levels. But unlike GLP-1, which has a half-life of just minutes, exendin-4 can last in the body for hours, providing a much more sustained response to glucose.
Its relevance to the treatment of type 2 diabetes — a disease that affects blood-glucose levels by compromising the body’s production and use of insulin — was apparent. A compound that could mimic GLP-1’s ability to stimulate insulin production but do so for a more sustained time period could theoretically help people with type 2 diabetes to regulate their blood-glucose levels better. Exendin-4 became exenatide (initially sold as Byetta), a synthetic drug that needed to be injected twice a day, and which was approved by the US Food and Drug Administration (FDA) in 2005 for the treatment of type 2 diabetes. In 2006, it earned manufacturer Amylin around US$430 million, and sales increased by nearly 50% the following year.
But it soon became evident that many people taking the drug were also losing weight.
As exenatide was being developed, researchers at Danish pharmaceutical company Novo Nordisk had been looking at the diabetes blood-glucose-control problem from a different angle. Recognizing that GLP-1 might be a useful target, but also aware that its short half-life in the body was its weakness, they instead focused on trying to make the hormone last for longer in the bloodstream. Novo Nordisk researcher Lotte Bjerre Knudsen and her colleagues found that if they attached a fatty-acid molecule to GLP-1, it extended the molecule’s half-life and was associated with even greater weight loss than exenatide.
That discovery led to the drug liraglutide — sold under the brand names Saxenda and Victoza — which still required daily injections. With the goal of achieving a once-weekly dosage level, Knudsen and her colleagues tweaked the structure of liraglutide to extend its longevity in the body. Finally, Novo Nordisk had a long-acting, stable version of GLP-1, which it called semaglutide.
The PIONEER-1 placebo-controlled study, published in 2019, showed that the drug was associated with significant improvements in blood-glucose levels in people with type 2 diabetes2. It also pointed to the possibility of significant weight loss when given at higher doses, with only mild to moderate gastrointestinal side effects.
Branching out
Novo Nordisk started selling semaglutide under the name Ozempic in 2017, for adults with type 2 diabetes with or without cardiovascular disease or kidney disease. But the weight loss seen in participants in the earlier type 2 diabetes trial had flagged even greater potential, so in 2015, Novo Nordisk launched a phase II, randomized, double-blind, placebo-controlled trial of semaglutide for weight loss in people with obesity.
Wilding was involved in that trial. “It was really exciting, because all of a sudden we were in a league where we knew that it would actually be much more impactful in terms of the effects on health,” Wilding says. The year-long trial, published in 2018, compared various daily doses of semaglutide or liraglutide with a placebo in nearly 900 people with obesity. It showed that between one-third and a half of those taking semaglutide lost at least 15% of their body weight, and at least three-quarters lost more than 5%3.

Wegovy (semaglutide) pre-filled injection pens are produced at the Novo Nordisk pharmaceutical manufacturing facility in Denmark.Credit: Charlotte de la Fuente/Bloomberg via Getty
Then, in 2021, came the Semaglutide Treatment Effect in People with Obesity trial (STEP 1), which found more than half the participants given weekly semaglutide injections over a period of 68 weeks achieved at least 15% weight loss, compared with around 5% in the control group, who received counselling about diet and exercise4.
A few months later, the FDA approved semaglutide, under the brand name Wegovy, as a weight-loss therapy for people who were overweight or obese.
Since then, there have been numerous clinical trials of semaglutide, confirming the weight-loss effect and pointing to other possible benefits. A study called STEP 9 suggested that the weight loss was associated with improvements in knee osteoarthritis pain5. Another study found a reduced risk of complications related to kidney disease in trial participants with diabetes6. Most recently, the SELECT trial found a 20% lower rate of fatal and non-fatal cardiovascular events among people who were overweight or obese and had a history of cardiovascular disease, but not diabetes7.
The precise mechanisms by which semaglutide and other GLP-1 receptor agonists achieve significant weight loss aren’t entirely understood, says molecular pharmacologist Sebastian Furness at the University of Queensland in Brisbane, Australia. “It acts in a part of the brain called the arcuate nucleus, which is sort of the control-command centre for appetite,” Furness says. “It’s very common that people taking Ozempic say it stops that little niggling thing in the back of my head saying ‘you’re hungry, go and eat’.”
GLP-1 is also released in the brain’s reward pathways, Furness says, so taking a GLP-1 receptor agonist such as semaglutide could also be modulating the reward associated with food intake. “There’s definitely evidence that in the reward pathway, GLP-1 — if you infuse it into the brain — is reducing that dopaminergic signalling which would normally be associated with reward,” he says. “But we don’t really know, it’s just a lucky outcome.”
The end of obesity?
Some question whether drugs like semaglutide are the solution to obesity. “If there’s this sort of magic pill, what does it do to all these social things that have happened that have brought us to this point in the first place; all the structural things in society that have brought us to this point?” says physician and sociologist Virginia Chang at the New York University School of Global Public Health.

Virginia Chang says the socio-economic factors of obesity must be considered.Credit: Chris Alexander
More than one billion people worldwide are living with obesity, and its prevalence more than tripled between 1975 and 2022. But obesity is not equally distributed across the population. In the United States, risk of obesity is higher among Black and Hispanic adults and people with low socio-economic status, driven by factors such as a lack of access to recreational spaces and affordable nutritious food, combined with an overabundance of low-cost fast foods.