Cloning the CROP-seq-CAR screening vectors
The design of the CROP-seq-CAR vectors builds on the CROP-seq technology2, which enables CRISPR genome editing with polymerase III-transcribed gRNAs and incorporation of the gRNAs into a separate polymerase II transcript for versatile detection in single cells. We designed a CROP-seq-CAR lentiviral transfer plasmid that uses the EF1α promoter to drive expression of the CAR, followed by a P2A self-cleaving peptide and a puromycin resistance marker. In CROP-seq, the gRNA is placed inside the lentiviral 3′ LTR, just before the polyadenylation signal of the vector. There, it becomes part of the highly expressed CAR-P2A-Puro transcript and is readily detected by bulk and single-cell RNA-seq assays. The gRNA cassette is also duplicated into the 5′ LTR during lentiviral integration and transcribed from a polymerase III promoter for effective genome editing. This way, CROP-seq-CAR vectors simultaneously achieve high genome-editing performance and an efficient sequencing-based readout of the gRNA.
The CROP-seq-CAR vectors were cloned from the CROPseq-Guide-Puro plasmid (Addgene, 86708), which was digested with XmaI and SalI. The resulting 7,737-bp fragment was extracted from a gel using the S.N.A.P. UV-Free Gel Purification Kit (Thermo Fisher Scientific, K200025) and dephosphorylated with Shrimp Alkaline Phosphatase (rSAP, NEB, M0371S) for 1 h at 37 °C, followed by heat inactivation for 20 min at 80 °C. We ordered two gBlocks (IDT) encoding EF1α-CAR (gBlock-17) and P2A-mCherry-WPRE (gBlock-18), with the CAR consisting of a leader peptide, the single chain variable fragment (scFv) of an antibody binding CD19 (clone FMC63), the transmembrane domain of CD8, the intracellular co-stimulatory domain of 4-1BB and the CD3ζ chain. The gBlocks were amplified by PCR using Q5 High-Fidelity 2X Master Mix (NEB, M0492L) with primers gBlock-17-FWD and gBlock-17-REV for gBlock-17 or with gBlock-18-FWD and gBlock-18-REV for gBlock-18. These primers extend the gBlocks with BsmBI type IIS restriction sites and overhangs for Golden Gate cloning. PCR-amplified and gel-purified gBlocks were digested with BsmBI-v2 (NEB, R0739S), column purified and ligated with the Quick Ligation Kit (NEB, M2200S). The ligation product was amplified by PCR with primers gBlock-17-FWD and gBlock-18-REV, column purified, digested one more time with BsmBI-v2 and inserted into the CROP-seq backbone by Gibson assembly using NEBuilder HiFi DNA Assembly Master Mix (NEB, E2621S). Sequences of the gBlocks and amplification primers are provided in Supplementary Table 1i. This cloning yielded the pPD0010_CROP-seq-CAR-mCherry2 vector (Addgene, 242533), from which all CROP-seq-CAR variants were derived.
For efficient selection of transduced cells, mCherry was replaced with the PAC antibiotic resistance gene, resulting in the pPD0039_CROP-seq-CAR-Puro vector (Addgene, 242532) used in most screens. To generate variants with different CARs, this vector was linearized by PCR using Q5 High-Fidelity 2X Master Mix (NEB, M0492L) and primers prCART0026 and prCART0028, which exclude the sequence for the CD19 scFv. Sequences for the scFvs for GD2 (14g2a scFv) and GPC3 were synthesized as gBlocks (IDT), amplified by PCR with primers adding overhangs that are homologous to the ends of the linearized backbone (sequences are provided in Supplementary Table 1i) using Q5 High-Fidelity 2X Master Mix (NEB, M0492L) and cloned by Gibson assembly using NEBuilder HiFi DNA Assembly Master Mix (NEB, E2621S). This resulted in the pCART0017_CROP-seq-CAR-Puro_GD2-BBz (Addgene, 242985) and pCART0016_CROP-seq-CAR-Puro_GPC3-BBz (Addgene, 242984) vectors. The pPD0035_CROP-seq-CAR-Puro_CD19-28z (Addgene, 242983) vector was generated by replacing the sequence for the intracellular 4-1BB co-stimulatory domain with the sequence for the CD28 co-stimulatory domain ordered as a gBlock (IDT).
Cloning the CROP-seq-CAR-multi screening vectors
The CROP-seq-CAR-multi vectors were cloned from the CROPseq-multi-v2-mTagBFP2-NLS plasmid (Addgene, 225754), which was amplified with primers CROPseq_FWD_016-minusACC and CROPseq-multi-v2_REV1 (Supplementary Table 1i) using Q5 High-Fidelity 2X Master Mix (NEB, M0492L) to generate the backbone for all variants of the CROP-seq-CAR-multi vectors (7,622 bp, including 40-bp extensions), followed by DpnI digestion (NEB, R0176L) to remove the parental plasmid. Sequences for the CARs were amplified from the corresponding CROP-seq-CAR vectors with primers PD39-FWD1 and CROPseq_REV_019 (Supplementary Table 1i), followed by DpnI digestion. PCR products were digested with PaqCI (NEB, R0745S), heat inactivated and cleaned with the Monarch PCR & DNA Cleanup Kit (NEB, T1030). The purified PCR fragments were ligated using T4 DNA Ligase (NEB, M0202T) at a 3:1 ratio of insert:backbone, resulting in the CROP-seq-CAR-multi vectors pCROP-seq-CAR-19-BBz-multi, pCROP-seq-CAR-19-28z-multi and pCROP-seq-CAR-GD2-BBz-multi.
Cloning the mRNA expression vectors
Open reading frames (ORFs) for mRNA production were cloned into the pIVTRup vector (Addgene, 101362) by Gibson assembly. The pIVTRup backbone was linearized by PCR with the primers pIVTRup_linearize_FWD and pIVTRup_linearize_REV (Supplementary Table 1i). ORFs for CRISPR modifiers and selection markers were amplified from existing plasmids (Supplementary Table 1b) with ORF-specific primers that included extensions for Gibson assembly: mRNA_ORF_Gibson_FWD and mRNA_ORF_Gibson_REV (Supplementary Table 1i). Both backbone and insert were amplified using Q5 Hot Start High-Fidelity 2X Master Mix (NEB, M0494L). Each 50-μl PCR reaction was mixed with 6 μl CutSmart Buffer, 3 μl nuclease-free water and 1 μl DpnI (NEB, R0176S) and incubated for 1 h at 37 °C to digest the plasmid template, followed by heat inactivation for 20 min at 80 °C. PCR products were cleaned with the QIAquick PCR Purification Kit (Qiagen, 28106). The final product was assembled with NEBuilder HiFi DNA Assembly Master Mix (NEB, E2621L) using a 2:1 molar ratio of insert:backbone. After incubating the mix for 15 min at 50 °C, the samples were stored at −20 °C or directly used for bacterial transformation. All plasmids were validated by Sanger sequencing.
Cloning of gRNAs and gRNA libraries
Individual gRNAs and small-scale gRNA libraries were cloned into CROP-seq-CAR vectors as described previously2. To clone the genome-wide Brunello gRNA library into the CROP-seq-CAR vector, we amplified the gRNA insert from Addgene plasmid 73178 by PCR31. We mixed Q5 Hot Start High-Fidelity 2X Master Mix (NEB, M0494L), SYBR Green, 0.5 µM of the primers gRNA_library_FWD and gRNA_library_REV and 1 µg of plasmid DNA per 50-μl reaction. The amplification was stopped at approximately 3,000 relative fluorescence units (RFU) in the exponential phase to avoid library overamplification. We treated the PCR product with DpnI (NEB, R0176L) to remove plasmid DNA and concentrated the sample by ethanol precipitation to achieve high purity. The Brunello gRNAs were assembled into the BsmBI-linearized CROP-seq-CAR vector using NEBuilder HiFi DNA Assembly Master Mix (NEB, E2621L) as described previously2.
Cloning of the gRNA library for in vivo CROP-seq
To prepare the gRNA library for in vivo CROP-seq (Supplementary Fig. 4a), a backbone fragment was amplified by PCR from the pPD0039_CROP-seq-CAR-Puro (Addgene, 242532) plasmid using Q5 High-Fidelity 2X Master Mix (NEB, M0492L) and 0.5 µM of the primers CART52_Bkbn_FWD and CART52_Bkbn_REV (Supplementary Table 1f). For quality control, we ran 1 µl of the PCR reaction on a 0.8% agarose gel containing SYBR Safe stain and verified the product size of 9,783 bp. Template plasmid was removed by DpnI digestion (NEB, R0176L) for 1 h at 37 °C, followed by inactivation of the enzyme for 20 min at 80 °C, cleaning with the QIAquick PCR Purification Kit (Qiagen, 28104) and quantification with the Qubit dsDNA HS assay (Thermo Fisher Scientific, Q32854). The gRNA library (libCART011, Supplementary Table 5b) was obtained as an oPool (IDT). The construct’s constant region (CART52_const_ultra, Supplementary Table 1f) was ordered as an ultramer oligonucleotide (IDT). The gRNA library oPool and the constant ultramer oligonucleotide were diluted to 2 µM and annealed in T4 DNA Ligase Buffer (NEB, B0202S). The library insert cassette was extended and amplified using Q5 High-Fidelity 2X Master Mix (NEB, M0492L) with 0.5 µM of the primers CART52_cassette_FWD and CART52_cassette_REV (Supplementary Table 1f). The product size of 225 bp was confirmed on a 2% agarose gel. The backbone and library insert were assembled by Gibson assembly using NEBuilder HiFi DNA Assembly Master Mix (NEB, E2621L). Gibson assembly reactions were desalted by filter dialysis (Merck, VMWP04700) and electroporated into Lucigen Endura Escherichia coli cells (Lucigen, 60242-2). The library plasmid was prepared using the Plasmid Plus Giga Kit (Qiagen, 12991).
Cloning of the gRNA library for combinatorial screening
For combinatorial screening, we selected the six strongest CAR T cell boosters based on the pooled in vivo screen (FAS, RHOG, PRDM1, CDKN2A, HAVCR2 and CTLA4) and three essential genes (RPL8, PSMB4 and POLR2L). For each gene, we chose the two best-performing gRNAs (of a total of eight). As negative controls, we selected the 15 most neutral PPP1R12C safe-harbour-targeting gRNAs, from which we randomly picked gRNA pairs (Fig. 5a and Supplementary Table 5b). The gRNAs that target boosters and essential genes were paired separately, yielding combinations targeting the same genes, different genes within each set or one target gene and the safe harbour locus. This resulted in 238 dual-gRNA combinations (Fig. 5a and Supplementary Table 7a), for which we designed a CROP-seq-multi library based on published source code (https://github.com/rtwalton/CROPseq-multi)46.
CROP-seq-CAR-multi library inserts (238 oligonucleotides, Supplementary Table 7a) were obtained as an oligonucleotide pool (Twist Bioscience) and amplified by PCR using 2× KAPA HiFi HotStart ReadyMix (Roche, KK2601) with 1 M betaine (MilliporeSigma, B0300), 300 nM primers (oRW1083 and oRW1086) and 12 pg µl−1 template per 25-µl PCR reaction. Thirty-three PCR reactions were set up with the following conditions: 95 °C for 3 min, 13 cycles of (98 °C for 20 s, 62 °C for 15 s, 72 °C for 15 s), 72 °C for 1 min. Here, the low input and few amplification cycles help reduce recombination during the PCR46. The pooled PCR products were purified using the Monarch PCR & DNA Cleanup Kit (NEB, T1030), digested with BsmBI-v2 (NEB, R0739L) and purified. The CROP-seq-CAR-multi vectors (pCROP-seq-CAR-19-BBz-multi, pCROP-seq-CAR-19-28z-multi and pCROP-seq-CAR-GD2-BBz-multi) were digested with BsmBI-v2, dephosphorylated using rSAP (NEB, 0371L) and purified. In a total volume of 20 μl, 20 fmol of digested oligonucleotide pool and 20 fmol of digested plasmid backbone were ligated using 400 U µl−1 of T4 DNA ligase (NEB, M0202S) at 16 °C overnight, followed by heat inactivation and cleanup with solid-phase reversible immobilization (SPRI) beads (Beckman Coulter, B23317) at a 1.5× ratio. The purified ligation product was electroporated into Lucigen Endura E. coli cells (Lucigen, 60242-2) with a Gene Pulser Xcell system (Bio-Rad, 1652662) (settings: 1.8 kV, 600 Ω and 10 µF), which were allowed to recover for 90 min at 30 °C in 1 ml of Endura recovery medium, followed by growth in a 100-ml liquid culture in selection LB medium (100 μg ml−1 carbenicillin) at 30 °C, with shaking at 225 rpm. Library plasmids were prepared using the Plasmid Plus Midi Kit (Qiagen, 12943).
Production of lentivirus
Lentivirus was produced using Lipofectamine 3000 (Invitrogen, L3000150) as described previously2, followed by 100× concentration using the Lenti-X Concentrator (Takara, 631232). For large-scale lentivirus production, all reagent and cell culture volumes were scaled accordingly for Nunc EasyFill Cell Factory Systems (Thermo Fisher Scientific). Lentivirus titre was quantified by quantitative PCR (qPCR) using the Lenti-X qRT–PCR Titration Kit (Takara Bio, 631235). For each lentivirus preparation, two aliquots of concentrated virus were diluted 100× with 1× PBS and used as input for quantification, and the mean titre (RNA copies per ml) was calculated.
Production of mRNA
CRISPR editors were cloned into our mRNA production vector as described above. This vector contains 5′ and 3′ untranslated regions to stabilize the mRNA, and the mRNAs are produced by in vitro transcription from a T7 promoter with cotranscriptional capping and terminated by a hardcoded A-tail of 55 bases. Optionally, modified bases such as 5-methoxy-U can be incorporated to optimize mRNA properties54. However, this increases the cost of mRNA production and was not necessary for efficient genome engineering in our screens.
To prepare for mRNA production, the corresponding DNA template was amplified by PCR from 10 ng of pIVTRup with the cloned ORF using Q5 Hot Start High-Fidelity 2X Master Mix (NEB, M0494L) and 0.5 μM of the primers pIVTRup_FWD_AG and pIVTRup_REV (Supplementary Table 1i) in a total volume of 50 µl and using 26 amplification cycles. The PCR product was cleaned using the QIAquick PCR Purification Kit (Qiagen, 28106). Synthetic mRNA was produced by in vitro transcription with the HiScribe T7 High Yield RNA Synthesis Kit (NEB, 2040S). Reactions contained 500 ng of PCR product, 10 mM of each nucleotide and 8 mM of CleanCap Reagent AG (TriLink BioTechnologies, N-7113-10) and were incubated for 2 h at 37 °C, followed by DNase I treatment (Promega, M6101) for 15 min at 37 °C. For large-scale production, the number of reactions was scaled accordingly. Immediately after the incubation, mRNA was cleaned with the RNeasy Mini Kit (Qiagen, 74104), loading 8–16 reactions per column. mRNA concentration was measured with a Qubit RNA BR Assay Kit (Invitrogen, Q33223), and mRNA integrity was assessed with the Bioanalyzer RNA 6000 Pico Kit (Agilent, 5067-1513).
T cell isolation
Peripheral blood samples from healthy donors were obtained through the Austrian Red Cross as blood packs with buffered sodium citrate as anticoagulant. Human primary T cells were isolated using RosetteSep Human T Cell, CD4+ T Cell or CD8+ T Cell Enrichment Cocktails (Stemcell, 15061, 15062 and 15063) to isolate pan-CD3+, CD4+ or CD8+ T cells, respectively. After incubation with the antibody cocktail, cells were centrifuged using Lymphoprep (Stemcell, 7861) in SepMate-50 tubes (Stemcell, 85460), washed and treated with 1× RBC Lysis Buffer (eBioscience, 00-4333-57). Isolated T cells were immediately stimulated and cultured as described below.
Patient-derived T cells were obtained from a 57-year-old female patient diagnosed with relapsed stage III multiple myeloma who previously received multiple lines of chemotherapy and autologous stem cell transplantation and was scheduled to receive anti-BCMA CAR T cell therapy as part of routine clinical care. The apheresis product for clinical CAR T cell production was collected using the Spectra Optia Apheresis System (Terumo BCT) with ACD-A as anticoagulant. T cells were isolated from leftover apheresis product using the EasySep Human T Cell Isolation Kit (Stemcell, 17951) following the manufacturer’s instructions for leukapheresis samples. Isolated patient T cells were immediately stimulated and cultured as described below.
The study complied with all relevant ethical regulations for working with human samples. Informed consent was obtained from all sample donors. The study was approved by the ethical committees of the contributing institutions (Austrian Red Cross and Medical University of Vienna).
T cell culture
T cell culture medium was based on CTS OpTmizer T-Cell Expansion Serum Free Medium (Thermo Fisher Scientific, A1048501): 1,000 ml of CTS OpTmizer T-Cell Expansion Basal Medium was supplemented with 26 ml of CTS OpTmizer T-Cell Expansion Supplement, 1% GlutaMAX (200 mM, 100×, Gibco, 35050061), 1% penicillin–streptomycin (10,000 U ml−1, Gibco, 15140122) and 2% human AB serum (heat inactivated according to the manufacturer’s instructions, Sigma, H4522). The complete medium was supplemented with 12.1 ng ml−1 human recombinant IL-2 (Stemcell, 78145.2) immediately before use. T cells were cultured at 37 °C with 5% CO2. For stimulation, T cells were seeded at 1 million cells per ml in T cell medium with 25 µl ml–1 ImmunoCult Human CD3/CD28 T Cell Activator (Stemcell, 10991). After 2–3 days of stimulation, the medium was exchanged completely. For collection, T cells were centrifuged at 400 rcf for 5 min. For optimization experiments, CD4+ T cells were frozen after 1 week of pre-expansion in CTS OpTmizer T-Cell Expansion SFM with 20% human AB serum and 10% DMSO. Immediately after thawing, cells were stimulated and cultured. Cells were counted using CASY TT (Schärfe System, 3222).
T cell transduction
T cells were collected 2–3 days after stimulation and seeded in fresh T cell medium at 1 million cells per ml. Lentivirus was added to the medium and mixed with cells. After 24–48 h, the medium was exchanged with a 4× larger volume. For single-locus targeting, 100× concentrated virus containing a single gRNA was added to the medium at a 1:10 (vol/vol) ratio. For screens, the lentivirus titre was quantified by qPCR, and lentivirus was added at 247 lentivirus RNA copies per cell for CRISPR screening in CD4+ T cells and at 432 copies per cell for CRISPR screening in CD8+ T cells.
T cell electroporation
For electroporation using the ExPERT ATx Electroporator (MaxCyte), cells were pelleted, washed once with room temperature 1× PBS, washed once with room temperature MaxCyte Buffer and then resuspended in MaxCyte Buffer at a concentration of 100 million cells per ml. Synthetic mRNA encoding CRISPR editors was added right before electroporation at a concentration of 150–300 μg ml−1. mRNA for fluorescent markers or the blasticidin resistance gene (encoding BSD) was added at a concentration of 75 μg ml−1. For RNP electroporation, synthetic gRNA (TrueGuide Synthetic gRNA, Synthego) and Alt-R S.p. Cas9 Nuclease V3 (IDT, 1081059) were mixed at a molar ratio of 2:1 and incubated for 30 min at room temperature for RNP complex formation. Immediately before electroporation, RNP was added to cells with a final gRNA concentration of 3 μM and a Cas9 concentration of 1.5 μM. Cells were mixed with mRNA or RNP and were transferred to MaxCyte cuvettes. Cuvettes were pulsed with a customized programme, Optimization 3-2. For electroporation using the Amaxa Nucleofector II system, cells were pelleted, washed once with room temperature 1× PBS and resuspended in 100 μl of Electroporation Master Mix from the Amaxa Human T Cell Nucleofector Kit (Lonza, 1002) immediately before electroporation at a maximum concentration of 15 million cells per ml and mixed with mRNA. Cells were transferred to the cuvettes and pulsed using the programme X-001. In both cases (electroporation with MaxCyte and Amaxa systems), cells remained in the cuvette for a 10 min recovery period and were then transferred to culture flasks with fresh prewarmed medium without puromycin at a concentration of 1 million cells per ml.
CRISPR editing of single genomic loci
T cells were freshly isolated or thawed from samples frozen after 1 week of pre-expansion. T cells were stimulated in T cell culture medium supplemented with 25 μl ml–1 ImmunoCult Human CD3/CD28 T Cell Activator (Stemcell, 10991). At 2–3 days after stimulation, cells were transduced with a lentivirus encoding the locus-specific gRNA (Supplementary Table 1c) and a fluorescent marker (mCherry) and the puromycin resistance gene (PAC). Selection with 1 μg ml−1 puromycin was started 2–3 days after transduction and maintained until the end of the experiment. Cells were restimulated 7–9 days after stimulation. Cells were co-electroporated 2–3 days later with mRNAs encoding a CRISPR editor and a fluorescent marker or the blasticidin selection gene. When using GFP or BFP mRNA as a co-electroporated selection marker, we gated successfully electroporated (BFP+ or GFP+) and transduced (mCherry+) live T cells by flow cytometry or FACS. When using the blasticidin resistance gene (encoding BSD) as the co-electroporated selection marker, cells were selected with 50 µg ml−1 blasticidin starting 24 h after electroporation, blasticidin was washed out after 1–2 days of selection and cells were further expanded for an additional 3–7 days.
CRISPR knockout and base editing
Cells were lentivirally transduced to express the locus-specific gRNA and electroporated with mRNA encoding Cas9, ABEmax, AncBE4max, ABEmax7.10-SpRY or CBE4max-SpRY (Supplementary Table 1b,c). After genome editing, cells were collected and genomic DNA was extracted. To measure editing efficiency at the DNA level, target loci were amplified using Q5 Hot Start High-Fidelity 2X Master Mix (NEB, M0494L) with locus-specific primers (Supplementary Table 1c). Amplicons shorter than 300 bp were sequenced directly. Longer amplicons were further fragmented with the Nextera XT Library Prep Kit (Illumina, 15032352). Libraries were sequenced on the Illumina MiSeq platform using MiSeq Reagent Kit v3 reagents (Illumina, MS-102-3001 or MS-102-3003). The frequency of insertions and deletions was determined based on the aligned sequencing reads. For CRISPR base editing, reads were considered successfully edited when they had at least one expected base conversion and no insertions or deletions. To assess editing at the protein level, we measured CD25 or CD44 levels by flow cytometry. For CD25, T cells were stimulated with 25 μl ml–1 ImmunoCult Human CD3/CD28 T Cell Activator for 24 h before flow cytometry to induce CD25 expression. For CD44, cells were cultured normally, given that CD44 is stably expressed in human T cells without need for stimulation.
CRISPR activation
Cells were lentivirally transduced to express a gRNA targeting the CD34 promoter. The gRNA backbone contained sequences for two MS2 aptamers for recruitment of the activators dCas9–VP64 and MCP–p65–HSF1, which were electroporated as separate mRNAs. Induction of CD34 surface protein was assessed by flow cytometry.
CRISPR screening
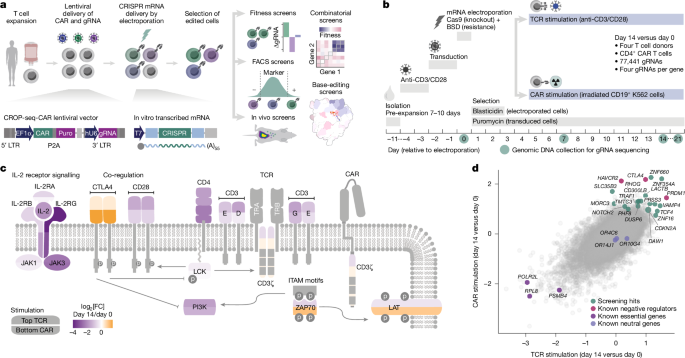
For genome-wide knockout screens and tiling base-editing screens, T cells (either pan-CD3+ or CD4+ only, depending on the experiment and documented in Supplementary Tables 2, 3 and 8) were freshly isolated, pre-expanded for 7–10 days and restimulated once more. For focused knockout and base-editing screens, T cells were freshly isolated or thawed from samples frozen after 1 week of pre-expansion and used immediately. T cells were stimulated with 25 µl ml−1 ImmunoCult Human CD3/CD28 T Cell Activator (Stemcell, 10991). At 2–3 days after stimulation, cells were transduced with CROP-seq-CAR lentivirus to deliver sequences for the CAR and the gRNA library and the puromycin resistance gene (PAC). At 2 days after transduction, cells were electroporated with mRNAs encoding the CRISPR editor and the blasticidin resistance gene (encoding BSD). Puromycin (0.5 μg ml−1) and blasticidin (50 μg ml−1) were added 24 h after electroporation. Puromycin selection was maintained until the end of the experiment. Blasticidin was removed after 24–48 h by a full exchange of cell culture medium. Cell numbers for transduction and electroporation were chosen to maintain at least 1,000× gRNA coverage (Supplementary Tables 2l and 3p).
For in vivo and combinatorial screens, CD4+ T cells were stimulated with 25 µl ml−1 ImmunoCult Human CD3/CD28 T Cell Activator (Stemcell, 10991). Two days after stimulation, cells were transduced with the lentivirus to deliver sequences for the CAR and the gRNA library and the puromycin resistance gene (PAC). Cells were subjected to puromycin selection (0.5 μg ml−1) 2 days after transduction. Selected CAR T cells were restimulated 5 days after transduction. CAR T cells were electroporated 2 days later with mRNAs encoding the CRISPR editor and BSD and further cultured.
Cancer cell lines
Antigen-expressing cell lines (K562-CD19 and K562-CD20) were produced by lentiviral transduction of K562 cells (ATCC, CCL-243) with the constructs pLenti-C-CD19-mGFP-P2A-Puro (OriGene, RC230267L4) or pLenti-C-MS4A1-mGFP-P2A-Puro (OriGene, RC221570L4). Cells were selected with 2 μg ml−1 puromycin (10 mg ml−1, Gibco, A1113802), which was maintained throughout cell culture. The suspension cell lines K562 and NALM6, the cell lines derived from them and firefly luciferase-expressing NALM6-GD2 cells were cultured in RPMI10 medium containing RPMI 1640 medium (Gibco, 11875085), 10% FBS (Sigma, F7524) and 1% penicillin–streptomycin (10,000 U ml−1, Gibco, 15140122). Cells were split, and culture medium was exchanged every 3–4 days. For genetically engineered cell lines, antibiotic selection was maintained throughout cell culture. The adherent cell line Huh7 was cultured in high-glucose DMEM (Sigma, D5796) supplemented with 10% FBS (Sigma, F7524) and 1% penicillin–streptomycin (10,000 U ml−1, Gibco, 15140122). Cells were split, and culture medium was replaced twice a week following standard procedures for adherent cells. For irradiation, cells were resuspended at 4 million cells per ml in RPMI10 medium and irradiated with 100 Gy using the Yxlon X-Ray System. Irradiated cells were centrifuged, washed once with fresh medium and either freshly used or frozen at 20 million cells per ml in freezing medium (RPMI 1640 (Gibco, 11875085) with 10% FBS (Sigma, F7524) and 10% DMSO (Sigma, D2650)). The K562, NALM6 and NALM6-GD2 cell lines were authenticated using the Cell Line Identification service by Eurofins. The Huh7 cell line was authenticated by the G. Superti-Furga laboratory. All cell lines used in this study were negative for Mycoplasma contamination throughout the study, which was ensured by regular testing using PCR.
Readouts of fitness screens
For TCR stimulation, cells were seeded at 1 million cells per ml in T cell medium containing 25 μl ml−1 ImmunoCult Human CD3/CD28 T Cell Activator (Stemcell, 10991) and cultured for 2–3 days before medium exchange and further culture. For CAR stimulation, cells were seeded at 1 million cells per ml in T cell medium containing 1 million irradiated K562-CD19 target cells per ml. The medium contained 0.5 μg ml−1 puromycin throughout and was exchanged every 2–4 days. On day 0 (before electroporation) and on days 7, 14 and 21 after electroporation, T cells were collected for gRNA sequencing. For screens of CD4+ CAR T cells, cells were lysed for DNA isolation immediately. For screens of CD8+ CAR T cells, cultures containing pan-CD3+ T cells were stained with live–dead stain and anti-CD8 antibody, fixed and purified by FACS to obtain a CD8+ CAR T cell population for genomic DNA isolation.
Readouts of FACS-based screens
For flow cytometry profiling, the BD LSRFortessa cell analyser was used. For cell sorting, the Sony SH800S cell sorter was used for optimization experiments and the BD FACSAria Fusion system was used for genome-wide screening. Cells were stained as follows: the cells were pelleted, washed with 1× PBS, stained with 1/1,000 Zombie Viability Dye (BioLegend) at room temperature for 10 min, washed and pelleted, stained with a mix of the relevant antibodies in Cell Staining Buffer (BioLegend, 420201) at 4 °C for 30 min, washed and pelleted, resuspended in the desired volume and filtered through a cell strainer. For storage before cell sorting, cells were fixed in FluoroFix Buffer (BioLegend, 422101) at room temperature for 30 min in the dark, followed by washing cells twice and storing them in Cell Staining Buffer at a concentration of 50 million cells per ml. For large-scale screens, staining and fixation steps were carried out in 50-ml tubes placed on a rotator to avoid cell pelleting and clumping. The staining reagents used in this study are listed in Supplementary Table 1h. Flow cytometry data were analysed using FlowJo v.10.810.0.
Amplification and sequencing of gRNAs
Genomic DNA was isolated using the DNeasy Blood & Tissue Kit (Qiagen, 69506) or the QIAamp DNA Blood Maxi Kit (Qiagen, 51194), depending on the number of cells. For cell lysis, we used AL buffer for freshly collected cells and ATL buffer for fixed and sorted cells. In both cases, cell lysates were incubated with proteinase K for 10 min at 56 °C, and fixed cells were additionally incubated for 4–12 h at 65 °C for DNA decrosslinking.
Library preparation for gRNA sequencing was performed as described in the CROP-seq protocol2. Up to 2 µg of purified genomic DNA was amplified and indexed with the primers CROPseq_libQC_i5 and CROPseq_libQC_i7 (Supplementary Table 1e), resulting in the sequencing-ready library. To avoid sequencing library overamplification, we added SYBR Green for qPCR quantification and stopped the amplification once it reached the exponential phase. For genome-wide screens, multiple PCR reactions were set up to retain 1,000× coverage for the individual gRNAs of the genome-wide gRNA library. Multiple 50-µl PCR reactions for the same sample were pooled together, and a minimum of 10% of the amplified material was used for purification. For plasmid libraries, we set up one to eight separate PCRs with 100 ng of input depending on the gRNA library size. We aimed for 1,000 sequencing reads per gRNA in the plasmid library. gRNAs amplified by PCR were cleaned by SPRI using Mag-Bind TotalPure Next-Generation Sequencing (NGS) Beads (Omega Bio-tek, M1378-01). Large DNA fragments were removed by cleanup with SPRI (0.45×) beads, followed by purification of the gRNA amplicon from the supernatant with a bead ratio of 1.0× to remove primer dimers.
Desired read numbers were calculated based on cell numbers and the estimated fraction of T cells with a gRNA, aiming for four to ten reads per cell. DNA concentrations for sequencing libraries were measured with the Qubit dsDNA HS assay (Thermo Fisher Scientific, Q32854), and 0.25 ng was analysed on a Bioanalyzer High Sensitivity DNA chip (Agilent, 5067-4626) to confirm the purity of the expected single band at 282 bp. Sequencing libraries were diluted to 3.5 nM with EB buffer containing 0.1% Tween and pooled according to the desired read number. Libraries were sequenced with 1% PhiX on the Illumina NovaSeq 6000 platform using a 100-cycle v1.5 flow cell with a read configuration of 122 bp for read 1, 8 bp for index 1 and 8 bp for index 2.
Library preparation for in vivo CROP-seq
Reverse transcription
RNA was isolated using the AllPrep DNA/RNA Mini Kit (Qiagen, 80204) and quantified with the Qubit RNA HS Assay Kit (Invitrogen, Q32852). For reverse transcription, 33 µl of RNA sample per reaction (maximum 15 µg) was mixed with 3 µl of 10 mM dNTPs (Thermo Fisher Scientific, R0193) and 3 µl of 2 µM CART0065_RT_002 primer (Supplementary Table 1f). To resolve RNA secondary structures, the reaction was incubated at 65 °C for 5 min and immediately placed on ice to prevent reformation. Next, a master mix of 12 µl of 5× SuperScript IV Buffer, 3 µl of 100 mM DTT (Sigma-Aldrich, 646563-10x.5ML), 3 µl RNaseOUT RNase inhibitor (Invitrogen, 10777019) and 3 µl SuperScript IV Reverse Transcriptase (Thermo Fisher Scientific, 18090010) was added. The reverse transcription reaction was incubated at 55 °C for 10 min, followed by heat inactivation for 10 min at 80 °C, and then placed on ice. Template RNA was digested with 3 µl RNase A (NEB, T3018L) for 30 min at 37 °C. Afterward, the cDNA was cleaned using 108 µl of Mag-Bind TotalPure NGS Beads (Omega Bio-tek, M1378-01), eluting in 40 µl nuclease-free water. Several reactions were carried out to ensure processing of the entire RNA isolated for each organ.
Nested PCR
The PCRs were set up with 19.5 µl template DNA, 25 µl Q5 High-Fidelity 2X Master Mix (NEB, M0492L), 2.5 µl of 10 µM FWD and REV primers and 0.5 µl of 100× SYBR Green. PCRs were incubated at 98 °C for 30 s (initial denaturation) and then cycled between 98 °C for 10 s and 72 °C for 40 s in a Bio-Rad CFX96 qPCR cycler until the reaction reached >1,500 RFU. PCRs were finished in a second cycler at 72 °C for 2 min and then cooled on ice. PCRs were cleaned with SPRI (0.8×) using Mag-Bind TotalPure NGS Beads (Omega Bio-tek, M1378-01). The outer PCR used primers CART0065 FWD-001 and CART0065 REV-001 (Supplementary Table 1f). The inner PCR used CROPseq_libQC_i5 with different stagger lengths and i5 indices, and TruSeq_i7 with different i7 indices. Primer sequences are listed in Supplementary Table 1g, and a schematic overview is provided in Supplementary Fig. 4a,b.
NGS library quality control and sequencing
Sequencing libraries were quantified with the Qubit dsDNA HS assay (Thermo Fisher Scientific, Q32854). A portion (1.5 ng) of the sequencing library was run on a Bioanalyzer High Sensitivity DNA chip (Agilent, 5067-4626) to verify the final size of 288–294 bp (depending on stagger length). Sequencing libraries were diluted to 2.0 nM with EB buffer containing 0.1% Tween and sequenced on the Illumina NovaSeq 6000 platform using 100-cycle v1.5 reagents with a read configuration of 50 bp for read 1, 8 bp for index 1, 8 bp for index 2 and 26 bp for read 2.
Library preparation for combinatorial screens
Amplification of plasmid gRNA library
Amplification of the plasmid library was performed as described previously46, using 2× NEBNext Ultra II Q5 Master Mix (NEB, M0544), 2.5 µl of 10 µM FWD and REV primers (Supplementary Table 1j), 0.5 µl of 100× SYBR Green and 100 ng plasmid template, with the following cycling conditions: 98 °C for 1 min, followed by ten cycles of 98 °C for 10 s, 67 °C for 10 s and 72 °C for 15 s, followed by 72 °C for 1 min. The PCR reactions were cleaned with 1× Mag-Bind TotalPure NGS Beads (Omega Bio-tek, M1378-01). Quality control and dilution were performed as described above.
Amplification of genomic DNA from combinatorial screens
DNA amplification was performed in a Bio-Rad CFX qPCR cycler to monitor amplification using 2× NEBNext Ultra II Q5 Master Mix (NEB, M0544), 2.5 µl of 10 µM FWD and REV primers (Supplementary Table 1j), 0.5 µl of 100× SYBR Green and 2.5 µg genomic DNA with the following cycling conditions: 98 °C for 1 min, 26–32 cycles of 98 °C for 10 s, 67 °C for 10 s and 72 °C for 15 s with plate reading, followed by 72 °C for 1 min. Amplification was stopped three cycles after SYBR Green enrichment detection to avoid overamplification and unwanted recombination events. The PCR reactions were cleaned with 1× Mag-Bind TotalPure NGS Beads (Omega Bio-tek, M1378-01). Quality control and dilution were performed as described above. The reference samples were collected on the day of electroporation (day 0). Five days after electroporation, cells were restimulated with irradiated target cells (K562-CD19 or NALM6-GD2) and cultured without puromycin (as NALM6-GD2 cells are not puromycin resistant). Samples were collected for genomic DNA extraction 12 days after electroporation.
Luciferase reporter cell lines
Synthetic DNA fragments encoding firefly luciferase, a P2A self-cleaving peptide and the zeocin resistance gene (Luc-P2A-Zeo, all human codon optimized) were ordered as gBlock (IDT). The lentiCas9-Blast plasmid (Addgene, 52962) was digested with EcoRI and XbaI to replace the insert for Luc-P2A-Zeo. Lentivirus was produced with the Lipofectamine 3000 reagent. NALM6 and K562-CD19 cells were lentivirally transduced by spinfection as described previously2 and selected for 2–4 weeks using zeocin.
Luciferase assay on microwell plates
CAR T cells were co-cultured with luciferase-expressing K562-CD19 cells for 18 h. Cells were transferred to a 384-well assay plate (Corning, 3707). For lysis and luciferase detection, we used the Steady-Glo Luciferase Assay System (Promega, E2510) and a PerkinElmer Victor x3 2030 Multilabel Reader.
CAR T cell production for mouse validation experiments
Gene knockout using mRNA electroporation
Freshly isolated CD3+ T cells were transduced with CROP-seq-CAR lentivirus containing a pool of eight gRNAs for each target gene. Four gRNAs were taken from the genome-wide screen, and four additional gRNAs were designed using the VBC score method55. Selected gRNAs usually had no (but up to a maximum of two) off-target sites with two or less mismatches based on Cas-OFFinder56 predictions (Supplementary Table 5h). Selection with puromycin (0.5 μg ml−1) was started 2 days later. Cells were restimulated 7 days after isolation. Cells were electroporated 3 days later with Cas9 and mRNA encoding BSD. Blasticidin (50 μg ml−1) was added 24 h later. On the following day, cell culture medium was exchanged, and cells were cultured for an additional 3 days. Cells were injected into mice 5–6 days after electroporation.
Gene knockout using RNP electroporation
Freshly isolated CD3+ T cells were stimulated with 25 µl ml−1 ImmunoCult Human CD3/CD28 T Cell Activator (Stemcell, 10991) for 2 days before electroporation with RNPs as described above, directly followed by transduction with CROP-seq-CAR lentivirus. Puromycin selection (0.5 μg ml−1) was started 2 days later. Cells were restimulated 7 days after isolation with 25 μl ml−1 ImmunoCult Human CD3/CD28 T Cell Activator (Stemcell, 10991). Cell culture medium was exchanged 3 days after restimulation, and cells were cultured for an additional 3–4 days. At 5–6 days after electroporation, the cells were either directly injected into mice or frozen in Bambanker freezing medium (Nippon Genetics, BB02). CAR T cells were thawed 1 day before injection into mice in the presence of 0.1 mg ml−1 DNase I (Sigma, 11284932001).
Mouse in vivo xenograft cancer models
Mice were bred and maintained under specific-pathogen-free conditions at the Medical University of Vienna, which is authorized for animal breeding and experimentation under license BMWFW-66.009/0403-WF/V/3b/2014. Experiments were performed at the Core Facility Laboratory for Animal Breeding and Husbandry of the Medical University of Vienna and the Preclinical Imaging Laboratory at the Department for Biomedical Research of the Medical University of Vienna using individually ventilated cages with controlled temperature (20–24 °C) and humidity (30–70%) and a 12-h light–dark cycle. Experiments followed established guidelines and were approved by the institutional ethical committee at the Department for Biomedical Research of the Medical University of Vienna according to license BMWFW-2020-0.605.586 granted by the Austrian Federal Ministry of Education, Science and Research (BMBWF). Investigators were not blinded to treatment status during animal experiments, as they also conducted the outcome assessment; however, stringent objective criteria were established for data collection and analysis to avoid any potential biases. The determination of group sizes and the power calculation were carried out according to the approved animal license (BMWFW-2020-0.605.586).
Leukaemia xenograft model
NSG male or female mice at 8–12 weeks of age were intravenously injected with 0.5 million firefly luciferase-expressing NALM6 cells (clone G5, ATCC, CRL-3273) or 0.5 million firefly luciferase-expressing NALM6-GD2 cells in 150 µl of 1× PBS. After 5 days, CAR T cells were administered by intravenous injection. For in vivo screens, female mice were injected with 1 million CAR T cells per mouse. For single-knockout validation, we injected male mice with 0.6 million CAR T cells in 150 µl of 1× PBS per mouse. Mice were tracked by bioluminescence imaging and weight measurements and were monitored for any signs of morbidity. All experiments complied with the humane and ethical end points stated in the animal license (BMWFW-2020-0.605.586), including maximum weight loss. Owing to the fast and uniform engraftment of NALM6 cells, no randomization was performed. To reduce biases introduced by spillover during bioluminescence imaging, we imaged treatment groups together whenever possible.
Solid tumour xenograft model
NSG female mice at 8–12 weeks of age were subcutaneously injected (right flank) with 1 million Huh7 cells in 100 µl of 1× PBS. After 20 days and with an average tumour volume of 0.3–0.4 cm3, CAR T cells were administered by intravenous injection. Tumour size was tracked by measurement with a precision caliper. Mice with Huh7 solid tumour xenografts were randomized before CAR T cell injection according to tumour size to ensure that all groups had an equivalent tumour load. All experiments complied with the humane and ethical end points stated in the animal license (BMWFW-2020-0.605.586), including maximum tumour size.
IVIS bioluminescence imaging
XenoLight d-Luciferin K+ salt (PerkinElmer, 122799) was dissolved at 30 mg ml−1 in sterile 1× PBS and injected intraperitoneally at a concentration of 150 mg per kg body weight. Mice were anesthetized with isoflurane, and bioluminescence imaging was performed 15 min after injection on the IVIS Spectrum (PerkinElmer, 124262) or the LagoX instrument (Spectral Instruments Imaging). Bioluminescence was quantified using Living Image Analysis Software v.4.7.3 (PerkinElmer) or Aura In Vivo Imaging Software v.4.5.0 (Spectral Instruments Imaging).
Organ dissociation
During extraction, organs and cells were kept on ice for the entire procedure. Spleens were rinsed with ice-cold 1× PBS with 5 mM EDTA and 0.1% BSA (PBS + EDTA + BSA) and smashed through a 70-μm cell strainer, and the resulting cell suspensions were collected in 50-ml tubes. Femurs from both hindlimbs were collected, and soft tissue was removed with scissors. Bone marrow was flushed with PBS + EDTA + BSA. Cells were filtered through a 70-μm strainer and transferred to 50-ml tubes. Cell suspensions from both organs were centrifuged at 500 rcf for 5 min and resuspended in 3 ml of 1× RBC Lysis Buffer (eBioscience, 00-4333-57) for 5 min to lyse red blood cells; the reaction was stopped by adding 20 ml of cold PBS + EDTA + BSA. Supernatants were filtered through a 70-μm cell strainer, and cells were counted using the CASY TT (Schärfe System, 3222) system.
RNA-seq profiling of CAR T cells
CAR T cells were produced from pan-CD3+ T cells as the starting material. CD4+ and CD8+ CAR T cells were isolated for bulk RNA-seq using the EasySep Human CD4 Positive Selection Kit II (Stemcell, 17852) and the EasySep Human CD8 Positive Selection Kit II (Stemcell, 17853). RNA was isolated using the Monarch Total RNA Miniprep Kit (NEB, T2010S), starting from at least 200,000 lysed cells, and the extracted RNA was stored at −80 °C in 300 µl Protection Reagent. RNA-seq libraries were prepared with the NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (NEB, E7760L) and sequenced with paired-end 50-bp reads on the Illumina NovaSeq 6000 platform.
Data preprocessing for the CRISPR screens
Sequencing data were preprocessed with a custom pipeline, starting from demultiplexed but unaligned BAM files provided by the Biomedical Sequencing Facility at CeMM. BAM files were converted to FASTQ format using bedtools v.2.30.0 bamtofastq. Reads were trimmed with cutadapt v.3.4, using the -g flag to specify the 5′ adapter and stagger (Supplementary Table 1e). For the in vitro screens, raw gRNA counts were obtained with the MAGeCK package v.0.5.9.4 using the mageck count command, specifying gRNA sequences for alignment with the -l option. For in vivo CROP-seq screens, we used MAGeCK2, cloned from the GitHub repository davidliwei/mageck2 and the mageck2 count command, providing the gRNA sequences with the -l flag and defining the UMI configuration with –umi secondpair,0,26. Finally, quality-control metrics were derived from MAGeCK log files, including total reads, mapped reads, gRNA alignment rate, gRNAs with zero counts, Gini index and read-trimming statistics.
Analysis of the genome-wide fitness screens
MAGeCK RRA analysis
Samples obtained after TCR or CAR stimulation on days 7, 14 or 21 after electroporation were separately compared with samples collected before stimulation (day 0). The analysis was performed with MAGeCK package v.0.5.9.4 using the mageck test command. The -k option was used to specify the raw gRNA count table in TSV format. Samples were provided with -t and -c flags. We used –remove-zero both –remove-zero-threshold 0 to remove gRNAs with zero reads in both of the two compared samples. For the normalization method, we used –norm-method control, and we used –control-gene to provide a set of previously described negative control genes for human samples (gene set NEGv1)57. Day 0 samples were selected for variance estimation with the –variance-estimation-samples option. Essential genes were defined with a stringent threshold of log2[FC] < −1.5 and FDR < 0.01. Gene set enrichment for gene ontology (GO) terms labelled BP (for Biological Process) was performed in R with the clusterProfiler package (v.4.0.0)58, using the function compareCluster with extra arguments fun = ‘enrichGO’, OrgDb = ‘org.Hs.eg.db’, keyType = ‘SYMBOL’ and ont = ‘BP’. Next, the similarity between nodes was calculated with the pairwise_termsim function from the enrichplot package using the default Jaccard similarity coefficient method. The network graph was plotted using the enrichplot::emapplot function with parameters showCategory = 100, node_scale = 10, min_edge = 0.3, cex_line = 0.5, cex_category = 10, pie = ‘Count’ and cex_label_category = 1.
MAGeCK MLE analysis
Samples obtained before (day 0) and after (day 14) TCR or CAR stimulation were compared in one joint analysis with MAGeCK package v.0.5.9.4 using the mageck mle command. Raw gRNA counts in TSV format were provided with –count-table, and the experimental design was defined with the –design-matrix option. In the design matrix, the day 14 samples were modelled as TCR stimulated or CAR stimulated (Supplementary Table 2j). We further used –norm-method control and provided a set of non-essential genes (gene set NEGv1)57 via the –control-gene option. The analysis was performed with –permutation-round 2. The MAGeCK MLE gene_summary.txt output file was read with R package MAGeCKFlute v.1.12.0 using the ReadBeta function. Normalizing β values against essential genes is important when samples in the screen have different proliferation rates, as it is the case for TCR- and CAR-stimulated versus unstimulated T cells (Fig. 1b and Extended Data Fig. 3d). We thus normalized β values with the NormalizeBeta function, specifying a set of essential genes with the arguments posControl (CEGv2) and method = ‘cell_cycle’. Finally, we selected screening hits that improved the function of both T and CAR T cells and of CAR T cells alone.
Analysis of the genome-wide FACS-based screens
The sequencing data from the genome-wide sorting screens for the markers CD19, CD69, FAS and the combination of PD-1, LAG3 and TIM3 were preprocessed as described above and filtered with quality-control thresholds of gRNA alignment ≥62.5%, ≥2 mapped reads per sorted cell and ≥100 mapped reads per gRNA in the library. The trimming auto-determination result of MAGeCK was zero for ≥96% of reads, confirming correct adapter annotation and usage. For the CD69 screens, each of the three positive populations (CD69+, CD69++ and CD69+++) and all their combinations were individually analysed with MAGeCK RRA, and we selected the CD69+ population for the main comparison, as it has the most consistent signal owing to higher numbers of sorted cells than for the CD69++ and CD69+++ populations. For the FAS screens, in which three negative populations (FAS−−−, FAS−− and FAS−) were sorted, the FAS−−− population showed the strongest enrichment of the screening marker and was selected for the main comparison. All analyses were performed with MAGeCK package v.0.5.9.4 using the RRA algorithm with the mageck test command. We used the options –remove-zero both –remove-zero-threshold 0 to remove gRNAs with zero reads in both of the two compared samples. We specified the raw gRNA count matrix with -k, test samples with -t and unsorted reference samples with -c. Furthermore, reference samples were also provided with –variance-estimation-samples. For read normalization, we used –norm-method median and provided a set of non-essential genes (gene set NEGv1)57 via the –control-gene option. All comparisons were run in –paired mode.
Genes were selected for inclusion in the in vivo CROP-seq experiment based on the results of the TCR and CAR fitness screens (normalized MAGeCK MLE β > 0.99) and the FACS-based screens (log2[FC] > 1.5, FDR < 0.01), with RNA levels in human CAR T cells serving as an additional filter (Extended Data Fig. 5n and Supplementary Table 6b).
Analysis of in vivo CROP-seq screens
Sequencing data from the in vivo CROP-seq screens were preprocessed as described above. Reads with UMIs containing an N were discarded. Different versions of the raw gRNA and UMI count table were prepared. For clonal tracking, the full UMI information was retained. For internal replicate analysis, only UMI bases 1 (four internal replicates), 1–2 (16), 1–3 (64), 1–4 (256) and 1–5 (1,024 internal replicates) were used. Finally, a table was prepared in which all UMI information was discarded. All tables were further processed with mageck-ibar (https://bitbucket.org/WeiLab/mageck-ibar.git). Screening hits were defined using a stringent threshold of log2[FC] > 1.5 and FDR < 0.01. To determine the number of T cell clones in each sample, we prepared a knee plot by visualizing log10[rank] against log10[reads] for each UMI. To find the inflection point that separates T cell clones from background noise, we modelled a sigmoid curve of form L/{1 + exp[−k × (x − x0)]} + b, where L is the maximum value, k is the steepness and b is the vertical offset, using the nls function from the R package stats. For modelling the log-transformed data, we disregarded the top 50 UMIs with the highest read count and all UMIs with fewer than ten reads. The coefficient x0 represents the inflection point that separates T cell clones from background noise.
RNA-seq data processing
RNA-seq read data (unaligned BAM files) were aligned to the hg38 genome assembly using an adapted STAR pipeline59. The resulting count matrix was split by cell type and time point, and all downstream analyses were performed for each dataset separately. The analyses and visualizations described here were performed using a publicly available Snakemake (7.21.0)60 workflow (v.1.0.1)61. Transcripts were filtered using the filterByExpr function from the R package edgeR (v.3.32.1)62 with the following parameters: group set to cell type and time point, min.count to 30, min.total.count to 50, large.n to 20 and min.prop to 0.8. Filtering reduced the number of analysed transcripts from 60,676 to 15,856. The data were normalized using conditional quantile normalization from the R package cqn (1.44.0)63. The parameters used for this normalization included gene length and GC content. The normalization results were log2 transformed for downstream analyses. Batch effects related to T cell donor were corrected for by using the reComBat method64 based on the log-transformed data. Normalization and batch correction were performed on the entire dataset for downstream visualization by PCA and visualization of predefined signatures.
Differential expression and gene set enrichment analysis
Differential expression analysis was performed on the quality-controlled, filtered and normalized transcript counts using limma (3.46.0)65 to fit a linear model for detecting statistically significant transcripts in RHOG-knockout compared with standard CAR T cells. We applied a publicly available Snakemake workflow (v.1.0.2)66. Briefly, we used lmFit to fit the model to the data and the Bayes command of limma with incorporated mean-variance trend to compute t-statistics. For each comparison, we used topTable to determine transcript-wise average expression, effect sizes (log2[FC]) and statistical significance (adjusted P values based on the Benjamini–Hochberg method). Furthermore, we calculated transcript scores for each transcript in each comparison using the formula −log10[P value] × sign(log2[FC]) for use by the downstream ranked enrichment analyses.
Gene set enrichment analysis was performed with a publicly available Snakemake workflow (v.0.1.1)67 using GSEA (https://www.genepattern.org/modules/docs/GSEAPreranked/1) with the prerank function from the GSEApy 1.0.3 package68. ‘GO biological process sets’ gene sets were selected (http://www.broadinstitute.org/gsea/msigdb). Enrichment scores (ESs) reflect the degree to which a gene set is overrepresented at the top or bottom of a ranked list of genes, and normalized ESs are ES scores normalized by mean ES across all comparisons for the dataset. The results of all queries were aggregated by method and database. Additionally, we filtered the results by retaining only the union of terms that were statistically significant (adjusted P value < 0.05) in at least one query. The aggregated results were visualized using hierarchically clustered heat maps and bubble plots. The union of the most significant terms per query was determined, and their effect size and significance were visualized as a hierarchically clustered bubble plot encoding both effect size (colour) and statistical significance (size), with statistical significance denoted by an asterisk. For the summary visualizations, P values were capped at an adjusted P value of 0.0001 to minimize the visual effect of genes with very low P values. Clustering of enriched terms was carried out using the R clusterProfiler package v.4.8.1 emapplot function58. Cell cycle-related genes were defined from a gene signature69.
Analysis of combinatorial screens
Combinatorial screening data were processed as follows: Raw reads were extracted from unaligned BAM files (samtools view with flag -f 64 for read 1 and -f 128 for read 2), joined by the read identifier (column1 or QNAME of the BAM), and CROP-seq-multi features (spacer1, iBAR1, spacer2 and iBAR2) were extracted based on their position. To produce a count matrix for downstream analyses (Supplementary Table 7c), we matched the combination of spacer1, iBAR1, spacer2 and iBAR2 to the combinatorial gRNA library (Supplementary Table 7a, zero mismatches allowed). We calculated additional quality-control metrics, such as percent of perfect matches to library features, fold difference between the 10th and 90th percentiles of library counts and percent recombination between spacer–iBAR pairs (Supplementary Table 7d). The processed data were analysed with MAGeCK MLE, using a design matrix modelling the 19-BBz, 19-28z and GD2–BBz CARs (Supplementary Table 7e), with options –permutation-round 10 –max-sgrnapergene-permutation 40. We normalized the data relative to dual safe harbour gRNAs with the –norm-method control and –control-sgrna options. We provide sample annotation (Supplementary Table 7b), gRNA counts (Supplementary Table 7c) and MAGeCK MLE output (Supplementary Table 7f).
Comparison of screening quality with published datasets
We obtained CRISPR screening data for primary T cells from Shifrut et al.22, Wang et al.20, Carnevale et al.15 and Freitas et al.16 and data for the Jurkat cell line from DepMap release 24Q4 (ref. 34). Details on the data sources are provided in Supplementary Table 4b. For datasets for which raw gRNA counts were available (Shifrut et al.22 and Freitas et al.16), we calculated log2[FC] between day 16 (Shifrut et al.22) or day 20 (Freitas et al.16) to the plasmid reference. For the Jurkat data, log2[FC] values between day 21 and the plasmid reference were directly available (AvanaLogfoldChange.csv), and, for Carnevale et al.15, we obtained log2[FC] values from the MAGeCK RRA output. For Wang et al.20, only β values but not the full MAGeCK MLE output were available; therefore, we reprocessed our own data with the same MAGeCK MLE settings. To compare the dropout of essential genes across screens while taking the variance into account, we used the strictly standardized mean difference (SSMD) metric, calculated as
$$\text{SSMD}\,=\,\frac{{\bar{X}}_{{\rm{e}}}-{\bar{X}}_{{\rm{ne}}}}{\sqrt{{s}_{{\rm{e}}}^{2}+{s}_{{\rm{ne}}}^{2}}},$$
where \({\bar{X}}_{{\rm{e}}}\) and \({\bar{X}}_{{\rm{ne}}}\) are the mean log2[FC] of essential and non-essential genes, and \({s}_{{\rm{e}}}\) and \({s}_{{\rm{n}}{\rm{e}}}\) are the corresponding standard deviations. We used sets of 684 essential genes (CEGv2) and 928 non-essential genes (NEGv1), both from the BAGEL2 publication57. Screens with an SSMD < 0.5 were flagged as low quality and excluded from the downstream hit analysis.
Comparison of screening results with published datasets
We obtained MAGeCK output files for all high-quality screens (SSMD > 0.5): Shifrut et al.22 (TCR fitness, CGS-21680), Carnevale et al.15 (regulatory T cell, TGFβ, TCR fitness, adenosine), Freitas et al.16 (IL-2, TNF; CAR fitness) and this study (TCR fitness, CAR fitness; FAS; PD-1, LAG3, TIM3; CD69; CD19). Most screens were processed with MAGeCK RRA, in which we defined significant hits as z-normalized log2[FC] > 2 and FDR < 0.05. For the TCR and CAR fitness screens in this study, we used MAGeCK MLE with β-value normalization for essential genes via MAGeCKFlute to control for the different proliferation rates between TCR- and CAR-stimulated T cells, with a selection threshold of normalized β > 0.99. For the heat map in Extended Data Fig. 4g, we included all significant hits except those from our fitness screens, for which the high number of significant genes required us to limit the selection to the genes tested in vivo.
Base-editing gRNA library design
We first designed all possible gRNAs for each target gene, including all exons and ±20 bp into each intron or UTR. Editing outcomes were annotated using the Base Editor Design Tool (https://github.com/mhegde/base-editor-design-tool#base-editor-design-tool)48. We excluded gRNAs containing stretches of four or more T nucleotides, which can lead to premature termination of gRNA transcription. We also excluded gRNAs with BsmBI cut sites because the PCR-amplified gRNAs were cloned with Gibson assembly, during which the backbone was cut by BsmBI.
For the PAM-less tiling screens of RHOG and PAC, all possible gRNAs were used. For essential genes, we designed gRNAs introducing specific mutation types, depending on the base editor: 50 splice site mutations for ABEmax, 50 splice site and 50 nonsense mutations for AncBE4max, 200 splice site mutations for ABEmax7.10-SpRY and 200 splice site and 200 nonsense mutations for CBE4max-SpRY. To that end, we filtered gRNAs introducing nonsense or splice site mutations in the first half of the transcript, where the target base is in positions 5–7 (which corresponds to the central part of the editing window). We selected essential genes according to their rank in the MAGeCK RRA analysis of our genome-wide fitness screens, with a limit of four gRNAs per gene. For control gRNAs targeting a safe harbour locus, we designed gRNAs such that 50% of them contained ≥1 A but no C and 50% contained ≥1 C but no A in the editing window, such that they could serve as nontargeting controls for C-to-T and A-to-G editors, respectively. Each group contained 25% gRNAs with an NGG PAM and 25% gRNAs with a non-NGG PAM (Supplementary Table 8a).
For the base-editing validation screens, we selected gRNAs based on the z score from the initial screen, including the 3.5% most enriched gRNA for RHOG and the 3.5% most depleted gRNA for PAC based on the average z score of the two donors and for each donor separately and additional safe harbour-targeting control gRNAs, resulting in a final library of 323 gRNAs (Supplementary Table 8e).
Base-editing analysis
For plasmid and pre-electroporation reference samples, sequencing reads were aligned to the gRNA library. As the gRNAs are integrated into the genome, they may be susceptible to self-editing by near-PAM-less base editors upon electroporation. Therefore, we created expanded reference files separately for the A-to-G and C-to-T editors, containing all original gRNA sequences and sequences resulting from self-editing in the editing window (gRNA positions 4–8). We aligned reads from base-edited samples to these references and produced gRNA-level count files (Supplementary Table 8c). log2[FC] values were calculated by comparing samples from day 14 (screening readout) and day 0 (pre-electroporation reference) using MAGeCK RRA with parameters –norm-method median, –remove-zero both, –remove-zero-threshold 0, –paired and –variance-estimation-samples, pairing samples by T cell donor (2 donors for the initial screening and five donors for the base-editing validation screens) and using samples from day 0 for variance estimation. For visualization, protein 3D structures were downloaded from the PDB, and the 15 amino acids corresponding to the most impactful base-editing gRNAs were drawn as stick representation using PyMOL. Hydrogen bonds were computed between the highlighted amino acids and the small-molecule reagents using the following PyMOL command: distance hbonds, small_molecules, top15_aas, mode = 2.
Base-editing validation
Base-editing validation screens
CD4+ T cells were lentivirally transduced with the CROP-seq-CAR vector containing a focused gRNA library for base-editing validation screens (Supplementary Table 8e) and electroplated with base editor mRNA as described above. gRNA amplification was performed as described above for all base editors. For genomic locus sequencing, ABEmax-edited CAR T cells were collected 2 and 12 days after electroporation. A 200-bp region of the RHOG locus that contains high-scoring gRNAs from the focused validation screens was amplified by PCR with primers containing overhangs compatible with TruSeq indexing (Supplementary Table 1c) using Q5 Hot Start High-Fidelity 2X Master Mix (NEB, M0494L), followed by SPRI (1.2×) bead purification using Mag-Bind TotalPure NGS Beads (Omega Bio-tek, M1378-01). The purified PCR fragments were indexed by PCR using TruSeq indexing primers (Supplementary Table 1d) to prepare them for sequencing on the Illumina NovaSeq 6000 platform.
Single-gRNA validation
CD4+ T cells were lentivirally transduced with CROP-seq-CAR vectors, each harbouring a single base-editing gRNA, targeting either the RHOG or PAC gene. After 7 days of puromycin selection including 2 days of restimulation, CAR T cells were electroporated with mRNA encoding the ABEmax base editor (Supplementary Table 1b). Three days after electroporation, a portion of cells was collected for genomic DNA extraction and the remaining cells were restimulated with 25 µl ml−1 ImmunoCult Human CD3/CD28 T Cell Activator (Stemcell, 10991) and further cultured in the presence of puromycin, until they were collected for genomic DNA extraction on day 12 after electroporation. To assess editing efficiency at the target loci on the DNA level, the RHOG and PAC loci were amplified by PCR using Q5 Hot Start High-Fidelity 2X Master Mix (NEB, M0494L) with locus-specific primers (Supplementary Table 1c). The resulting amplicons from samples collected on day 3 and day 12 after electroporation underwent Sanger sequencing, and editing efficiency was analysed using the EditR tool70 with default parameters.
Material availability
Plasmids generated in this study, including the CROP-seq-CAR and mRNA production vectors, have been deposited at Addgene (Supplementary Table 1).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.