aSyn expression
Escherichia coli strain BL21(DE3) was transformed with pT7-7 aSyn WT vector43 from Addgene, (plasmid 36046) and plated onto a Luria broth (LB) agar plate containing ampicillin (100 μg ml−1) and 1 g l−1 glucose. A preculture in 5 ml of LB medium was inoculated with one clone and incubated at 37 °C under 200 rpm shaking for 4 h. Cells from the LB preculture were recovered by centrifugation (1,000g, 10 min) and used for inoculating 200 ml of LB medium. Cells were grown overnight at 37 °C under 200 rpm shaking and then diluted in 2 l of culture. Protein expression was induced by adding 0.5 mM isopropyl-β-d-thiogalactopyranoside during the exponential phase, evaluated at an optical density at 600 nm of 0.6. Cells were collected after 5 h of culture at 30 °C by centrifugation at 7,000g and 4 °C for 20 min (JLA 8.1000, Beckman Coulter), and the pellet was kept at −20 °C until purification.
aSyn purification
The pellets were resuspended in lysis buffer (10 mM Tris and 1 mM EDTA (pH 7.2) with protease inhibitor tablets (cOmplete, EDTA-free protease inhibitor cocktail, Roche)) and sonicated at 50% maximum energy, 30 s on and 30 s off for three rounds with a probe sonicator (Q-Sonica). The sonicated pellets were centrifuged at 20,000g for 30 min, and the supernatant was saved. The pH of the supernatant was then reduced to pH 3.5 using HCl, and the mixture was stirred at room temperature for 20 min and then centrifuged at 60,000g for 30 min. The pellets were discarded. The pH of the supernatant was then increased to pH 7.4 with NaOH and then dialysed against 20 mM Tris-HCl (pH 7.4) and 100 mM NaCl buffer before loading onto a 75 pg HiLoad 26/600 Superdex column equilibrated with the same buffer on the ÄKTA pure system. Monomeric fractions were collected and concentrated if needed by using the Vivaspin 15R 2 kDa cutoff concentrator (Sartorius Stedim). Purification fractions were analysed by polyacrylamide gel electrophoresis (PAGE) Tris-tricine 13% dying with ProBlue Safe Stain. The protein concentration was evaluated spectrophotometrically on the basis of the absorbance at 280 nm and an extinction coefficient of 5,960 M−1 cm−1. Quantification of the preparations using the Pierce chromogenic LAL kit indicated a low endotoxin (lipopolysaccharide, LPS) residual value of 0.03–0.06 EU μg−1 of recombinant protein.
aSyn fibrillization
Solutions of monomeric aSyn at 4 to 5 mg ml−1 in saline (H2O, 100 mM NaCl and 20 mM Tris-HCl pH 7.40) were sterilized by filtration through 0.22-μm Millipore single-use filters and stored in sterile 15 ml conical Falcon tubes at 4 °C. Sterilized stock was then distributed into safe-lock Biopur individually sterile-packaged 1.5 ml Eppendorf tubes as 500 μl aliquots and were seeded with 1% of 1B strain fibrils13,14,15. The tubes were cap-locked and additionally sealed with parafilm. All of the previous steps were performed aseptically in a particle-free environment under a microbiological safety laminar flow hood. The samples were loaded in a ThermoMixer (Eppendorf) in a 24-position 1.5 ml Eppendorf tube holder equipped with a heating lid. The temperature was set to 37 °C with continuous shaking at 2,000 rpm for 4 days. 1B templating of the fibrillization products was quality-checked using the fibrilloscope13,14,15.
Sonication
Before intracerebral injections, 1B aSyn fibril stocks (4 mg ml−1) were distributed in cap-locked, sterile 0.5 ml PCR tubes (Thermo Fisher Scientific). Sonication was performed at 25 °C in the Bioruptor Plus water bath sonicator (Diagenode) equipped with thermostatic control and an automated tube carousel rotator. The sonication power was set to high, and 10 cycles of 30 s on followed by 10 s off were applied. In agreement with our previous quantifications14, over 80% of the fibrils were 50-nm long or less after application of this protocol (not shown).
In vivo aSyn pathology
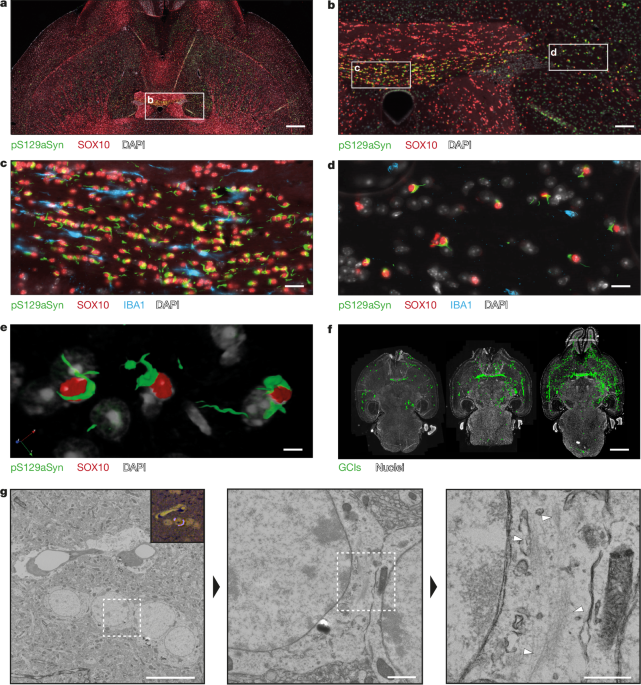
Adult 129SV (intrastriatal injections), C57BL/6 (nigral injections) and transgenic M83 (hemizygous, intrastriatal injections) mice were housed in a temperature-controlled (22 °C) and light-controlled environment under a 12 h–12 h light–dark cycle with forced ventilation (humidity below 50%) and with access to food and water ad libitum. All of the experimental procedures were conducted in accordance with the European Communities Council Directive (2010/63/EU) for care of laboratory animals. The study design was approved by the ethics committees of the University of Bordeaux, of the ANSES/Ecole Nationale Vétérinaire d’Alfort/Université Paris-Est Créteil and of the University of Salento, and authorized by the French Ministry of Higher Education and Research and by the Italian Ministry of Health (APAFIS 33147-2021091711598830 v6, APAFIS 37712-2022061615206629 v8, 0013178-P-17/05/2019). The mice (aged 6–8 weeks) unilaterally received 2 μl of sonicated aSyn fibrils 1B (4 mg ml−1) by stereotactic delivery at a flow rate of 0.4 µl min−1, and the pipette was left in place for 5 min after injection to avoid leakage. Delivery was performed within the right striatum (coordinates from bregma: anteroposterior (AP), −0.1; lateral (L), +2.5; dorsoventral (DV), +3.8), or above the right SN (coordinates from bregma: AP, −2.9; L, −1.3; DV, −4.5). WT mice (n = 20) and transgenic M83 hemizygous mice (n = 28) injected with either PBS, 1B fibrils or brain homogenates were euthanized after 6 weeks, 17 months, 24 months or when reaching the humane end points defined as death regarding the transgenic M83 mice, and were transcardially perfused with either Tris-buffered saline (pH 7.4) followed by 4% paraformaldehyde in PBS pH 7.4 at 4 °C, or with cacodylate buffer 0.1 M with 1 mM CaCl2 (pH 7.4) followed by 4% paraformaldehyde plus 0.1% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4). Brains were subsequently postfixed in the same fixative. For standard neuropathology, the brains were paraffin embedded, and 10-µm-thick sections were cut using a rotative microtome (Leica). The sections of interest were deparaffinized and processed for epitope retrieval: the slides were immersed in citrate buffer pH 6 (Dako Agilent Technologies) and placed into a pressure cooker (Bio SB) at 114–121 °C for 10 min. After a cooling period of 20 min, the slides were washed twice for 5 min in PBS at room temperature. The slides were then processed for simple or double immunofluorescence using the following primary antibodies diluted at 1:500, or their combinations: EP1536Y (Abcam) or pSyn#64 (Wako) for detecting pS129aSyn-positive inclusions; LB509 for detecting human aSyn (Abcam, mouse monoclonal); anti-SOX10 (Abcam, rabbit monoclonal) for detecting OL nuclei; EP1532Y and anti-TH raised in chicken (anti-tyrosine hydroxylase, Abcam) for detecting the nigrostriatal tract; and anti-IBA1 (Abcam) for detecting microglia. DRAQ7 or DAPI were used to image the nuclei. The AlexaFluor-coupled secondary antibodies were from Thermo Fisher Scientific (Alexa 488, 568 and 674). The sections were acquired using a Pannoramic slide scanner (3D HISTECH, MM France) in epifluorescence mode, and multichannel fluorescence optical sections of the samples were performed (thickness, <0.8 µm) using either a Zeiss CD7 platform of a Leica SP5 laser-scanning confocal microscope equipped with a spectral detector, 488, 561 and 633 nm laser lines, a motorized xy stage and a mixed stepping motor/piezo z controller. The objective was ×40, and the z step size was set to 0.5 µm to produce stacks of 15 to 20 z planes. The pinhole was set to 1 airy unit. For 3D reconstructions and volume rendering/animations (corresponding to 360° tilt series of composite maximum pixel projections images), raw three-channel z-stack images were processed offline using Icy44 (v.2.4.0.0).
HCA
Timed inbred-pregnant C57BL/6JOlaHsd (aSyn-knockout) female mice were received from Envigo 2 days before initiation of the primary culture. The pregnant mouse was euthanized by cervical dislocation, and the aSyn-knockout embryos (embryonic day 18) were surgically extracted and cold-euthanized. Cortices were collected from eight of them, pooled and dissociated enzymatically and mechanically (using a neural tissue dissociation kit, C Tubes and an Octo Dissociator with heaters; Miltenyi Biotech) to yield a homogenous cell suspension pool of eight individuals. The cells were then plated at 20,000 per well in 96-well plates (Corning, BioCoat poly-d-lysine imaging plates) in neuronal medium (MACS Neuro Medium, Miltenyi Biotech) containing 0.5% penicillin–streptomycin, 0.5 mM alanyl-glutamine and 2% NeuroBrew supplement (Miltenyi Biotech). The cultures were maintained under 5% CO2 at 37 °C in a humidified atmosphere. The medium was changed by one-third every 3 days, until 30 days in vitro (DIV). In such cultures, and under control conditions, neurons represented approximately 85 to 95% of the cell population; thus, for simplicity, they are here referred to as neurons. After 7 DIV, vehicle and sonicated 1B aSyn fibrils were added at a final concentration of 10 nM equivalent monomeric concentration. When relevant, neurons were infected at DIV 10 with AAV (adeno-associated virus) particles (multiplicity of infection, 1,000) carrying the cDNA of aSyn and its variants under the control of the human synapsin promoter, as previously described14. High content analysis (HCA) was performed after fixation and double immunofluorescence staining with the conformation-dependent SynF1 antibody (mouse monoclonal, BioLegend) and the human aSyn-specific MJFR1 antibody (rabbit monoclonal, Abcam) as previously described on images acquired at ×20 using the generic analysis module of an Incucyte S3 (Sartorius) system with Top-Hat segmentation14. For quantifications, the signal integral of the segmented areas in each field of view was recorded. Primary neuronal cultures from C57BL/6 embryos physiologically expressing endogenous mouse aSyn and not transduced with any AAV vector (Extended Data Fig. 1) were produced and analysed similarly but after fixation at DIV 24 and after revealing pS129aSyn with EP1536Y and human aSyn with LB509 antibodies.
CLEM analysis
The CLEM protocol was performed as previously described26,27. In brief, sections (thickness, 20–40 µm) were cut from perfused mouse brain on a vibratome (Leica VT1200). Immunolabelling against aSyn pathology allowed for the identification of immunopositive sections to be used for further processing with EM. For this, the sections were incubated overnight with a primary EP1536Y (Abcam) antibody at 4 °C followed by Alexa-conjugated secondary antibody staining together with DAPI for cell nuclei identification. Fluorescence images were acquired using the Leica Thunder Tissue Imager equipped with the K8 fluorescent camera. Chosen sections with strong aSyn pathology were prepared for EM by post-fixing in reduced 2% osmium, thiocarbohydrazide and 2% osmium tetroxide, followed by contrasting in 2% uranyl acetate and lead aspartate treatment at 60 °C. The sections were dehydrated with an increasing acetone gradient, before infiltration with EMBED 812 resin for final flat embedding. The hardened sections were imaged again using light microscopy and the sections were overlayed with the overview fluorescence map by using identifiable tissue features. Regions of interest were marked and the coordinates used for laser dissection. The resulting blocks were laser cut using the Leica LMD7 system and glued onto a resin block. Serial sectioning with an ultra-microtome (Leica UC7) produced 120–200-nm-thick tissue sections that were alternatingly collected on EM grids (slot and hexagonal grids) or glass slides. The glass slides were further processed for IHC and Toluidine Blue staining.
Light-microscopy images were acquired at ×40 magnification and correlated to each other for the generation of an image overlay of pathology signal (IHC) and high-contrast tissue morphology (Toluidine Blue). The resulting overlays were then correlated to low magnification (×640 nominal magnification) EM overviews for cell identification by using identifiable tissue features both in the LM and EM images. The identified pathology was finally imaged and merged at high magnification in a tile acquisition series. For EM image acquisition, a Thermo Fisher Scientific Talos F200C with a CETA camera or a CM100 (Phillips) with a TVIPS F416 camera were used. Light-microscopy and EM images were adjusted for brightness and contrast where necessary.
On-grid immunogold labelling and analysis
EM grids with tissue sections were placed on a droplet of etching solution (1% periodic acid in water, Sigma-Aldrich), with the sections facing down, for 2.5 min. The grids were washed three times for 2 min in double-distilled H2O, then washed twice for 2 min in washing buffer (stock solution of 10% BSA, Aurion, which was 1:50 diluted in double-distilled H2O), and blocked in blocking solution (Aurion) for 5 min to reduce non-specific binding. The grids were then incubated with primary antibodies (EP1526Y, Abcam, 1:50 diluted in washing buffer) for 60 min, washed 6 times for 2 min in blocking buffer and then incubated in a solution of gold nanoparticles with a size of 10 nm (protein A gold, Aurion, 1:50 diluted in washing buffer) for 90 min. They were washed three times for 2 min in blocking solution, three times for 2 min in washing buffer and four times for 1 min in double-distilled H2O. Finally, the grids were post-stained in 1% uranyl acetate for 1 min and washed three times for 10 s in double-distilled H2O. The sections were imaged using a transmission electron microscope (Thermo Fisher Scientific, Talos L120C), operated at an operating voltage of 120 kV, and images were recorded using a Thermo Fisher Scientific Ceta camera.
Gold particles were detected automatically in the recorded EM images, using FIJI45, by applying a difference-of-Gaussian filter (sigma1 = 10 nm, sigma2 = √2 × sigma1). The resulting image was binarized with a manually selected threshold. Particle detections with an area smaller than 25 nm2 and a circularity (4π × area/perimeter2) below 0.8 were excluded from analysis. The local density of gold was calculated as the decadic logarithm of the sum of a pixel’s distance to the 15 closest neighbouring gold particles. The gold particle detection was implemented as a FIJI macro and the density calculation as a Python notebook.
Electron tomography and 3D reconstruction
Electron tomography data were collected using the Tomography software (Thermo Fisher Scientific) with multisite batch acquisition on the Thermo Fisher Scientific Talos F200C system with tilt series covering −60° to +60° in 2° steps. Tomograms were reconstructed using IMOD46 and filtered with a non-local-means filter with Amira (Thermo Fisher Scientific, Amira v.2021.2). For segmentation, nuclear membranes, fibrils and other membranous parts were manually annotated and traced throughout the tilt-series using Amira. Every few slices, the respective feature of interest was selected by drawing directly onto the image and then interpolated to generate an accurate 3D volume. The final volume was visualized directly in AMIRA.
aSyn purification from brain homogenate
Extraction of the sarkosyl-insoluble fraction of a transgenic M83+/− mouse brain 16 weeks after inoculation with 1B was done using the same procedure as was previously published for fibrils purified from a patient with MSA11. In brief, brain tissue was homogenized in 20 vol. (v/w) extraction buffer consisting of 10 mM Tris-HCl, pH 7.5, 0.8 M NaCl, 10% sucrose and 1 mM EGTA. Homogenates were brought to 2% sarkosyl and incubated for 30 min at 37 °C. After a 10 min centrifugation at 10,000g, the supernatants were centrifuged at 100,000g for 20 min. The pellets were resuspended in 500 μl g−1 extraction buffer and centrifuged at 3,000g for 5 min. The supernatants were diluted threefold in 50 mM Tris-HCl, pH 7.5, containing 0.15 M NaCl, 10% sucrose and 0.2% sarkosyl, and centrifuged at 166,000g for 30 min. Sarkosyl-insoluble pellets were resuspended in 100 μl g−1 of 30 mM Tris-HCl, pH 7.4.
Immunogold labelling and negative staining of sarkosyl purification
A total of 3 µl of non-diluted sample was applied to 100 mesh Ni grids with formvar support (Electron Microscopy Sciences) that were glow-discharged at 15 mA for 60 s. After a 10 min incubation time, the grid was blotted, washed three times for 5 min with incubation solution (0.2% BSA-c/PBS; Aurion) and incubated for 1 h at room temperature in a solution of pS129aSyn antibody (EP1536Y, Abcam, ab51253) diluted 1:100 in incubation solution. The grid was washed six times for 5 min and incubated for 2 h in a solution containing gold nanobeads (diameter 6 nm) coupled to an anti-rabbit antibody (Aurion), diluted 1:50 in incubation solution. After washing six times for 5 min in incubation solution and three times for 5 min in PBS, the grid was immersed in 1% uranyl acetate for 40 s, blotted and air-dried. The grid was imaged on the Talos transmission electron microscope at an operating voltage of 200 kV (Thermo Fisher Scientific).
Cryo-EM analysis
Recombinant 1B fibrils
Cryo-EM grids were prepared using the Leica GP2 plunge freezer in a BSL-2 environment at room temperature. Quantifoil 300-mesh gold grids were glow-discharged in air for 90 s. Grids were prepared using 3 µl of 1:1 and 1:2 diluted fibril samples in preparation buffer (H2O, 100 mM NaCl and 20 mM Tris-HCl, pH 7.40) and vitrification by rapid freezing in liquid ethane. The grids were screened for the presence of non-clustered fibres and high-resolution images were acquired with an automated EPU (v3.10) setup on the Thermo Fisher Scientific Titan Krios G4 electron microscope equipped with a cold-FEG 300 kV electron source and a Falcon4i camera. In total, 11,879 dose-fractionated videos (EER) were collected with a total dose of 40 e− Å−2 at a pixel spacing of 0.66 Å at the sample level. A defocus range of between −0.8 μm and −1.8 μm was used during the acquisition.
Purified 1BP fibrils
The sarkosyl-insoluble fraction of a transgenic M83+/− mouse brain was diluted 1:3 in Tris-HCl, 50 mM, 150 mM NaCl, pH 7.4 and 2.5 µl was applied to a Quantifoil 300-mesh gold grid, coated with an additional 5-nm-thick carbon layer. The grid was not glow-discharged. Then, 30 s after applying the sample, the grid was blotted from the front side for 6 s and plunge-frozen at room temperature and 80% humidity in a Leica GP2 plunge freezer. A total of 42,812 exposures was collected on the Titan Krios (Thermo Fisher Scientific) electron microscope equipped with a cold-FEG 300 kV electron source and a Falcon4i camera. The total dose applied per video (EER) was 50 e− Å−2 and the pixel spacing was 0.732 Å. A defocus range of between −0.8 μm and −2.2 μm was used during the acquisition.
Helical reconstruction and model building of recombinant 1B fibrils
All the subsequent image processing and helical reconstruction was carried out in RELION (v.4.0)47 (Extended Data Fig. 13). The recorded videos were gain-corrected, motion-corrected and dose-weighted using RELION’s own implementation of MOTIONCOR. The raw EER files were dose-fractionated to a total of 32 frames (1.23 e− Å−2 per frame). After CTF estimation using Ctffind (v.4.1)48, the aligned averages that had an estimated CTF resolution of better than 5 Å were selected, resulting in a total of 11,879 micrographs. aSyn fibres were picked manually as start-to-end helical segments and extracted with an interbox distance of 19 Å. Several rounds of reference-free 2D classification were performed to exclude suboptimal 2D segments from further processing. The four best classes were selected and binned four times to re-extract the segments with a bigger box size encompassing the entire helical crossover. Clearly visible twisted class averages containing the entire crossover were used to generate initial de novo models using the relion_helix_inimodel2d program. The most likely twist was estimated from a crossover distance measured from 2D class averages and corroborated with the direct measurements from micrographs.
The helical segments (105,496) from the entire dataset were then re-extracted with a 600 pixel box size (binned by 2) comprising about 40% of the crossover. Exclusion of the suboptimal classes after 2D classification resulted in 64,889 particles. Multiple rounds of 3D autorefinement were carried out to optimize the helical twist and rise, and to check for the correct symmetry operators. This was validated from the reconstructions showing clear separation of β-strands along the helical axis. Imposing a 21 cork-screw symmetry (pseudo two-fold symmetry) yielded the clear separation of β-strands when compared to C1 and C2 symmetries. A 3D classification without image alignment was further performed to exclude the segments that gave suboptimal 3D volumes. This resulted in a final 51,272 particles. These were finally re-extracted at a 512 pixel (unbinned) box size and subjected to 3D autorefinement, followed by CTF refinement. Further iterations of Bayesian polishing coupled with CTF refinement yielded a map at 1.94 Å. The maps were sharpened using the standard post-processing procedure in RELION with an ad hoc B factor of 21 Å2.
The atomic model for the backbone of the core of the 1B fibrils encompassing residues 34 to 95 was built de novo using Coot49 (v.0.9.8.96) Initially, three β-rungs were modelled in coot and were refined in real-space in PHENIX45 (v.1.21.2) Finally, these chains were extended to 9 β-rungs per protofilament and refined in tandem with phenix.real_space_refine and in coot to improve the Ramachandran and Geometric statistics. NCS and secondary structural restraints were included during the iterative refinement process.
Helical reconstruction and model building of purified 1BP fibrils
As most of the 42,812 collected videos did not contain any fibrillar material, they were first imported into cryoSPARC50 (v.4.7.0), motion corrected and manually inspected using a manually_curate_exposures job. After manual inspection, 156 videos with intact, twisting fibrils were selected for further processing in RELION447. They were dose-fractionated in RELION’s MOTIONCOR implementation with 24 frames per fraction, corresponding to a dose of 1.28 e− Å−2 per frame, and CTF-corrected using Ctffind (v.4.1)48. Fibrils were found to cluster into three morphological groups (Extended Data Fig. 8). Manual picking was done in three rounds, using 209, 7 and 40 videos for the three respective groups. After extraction with binning of 2 and an inter-box distance of 14.4 Å (3 asymmetrical units), several rounds of 2D classification were applied. One of the three morphological groups (40 videos) yielded 2D classes for which a clear twist and monomer separation was visible, while the other two morphological groups did not yield any interpretable result.
Using the 2D class averages of the one usable group, a de novo initial model was generated using relion_helix_inimodel2d. Particles were re-extracted with a box size of 800 and a binning of 2 and, after multiple rounds of 2D classification, 1,913 particles were retained for a 3D classification job with 1 class and the de-novo inimodel2d reconstruction as initial model. When a fibril backbone was visible, the regularisation parameter (T) was gradually increased to 12 over the course of 50 iterations, and the result was used for a 3D refinement with a tau2 fudge factor of 2 to search for the optimal helical rise and twist. After CTF refinement and postprocessing, a 3D map at a resolution of 3.8 Å was obtained.
To build the 1BP atomic model, one β-rung of the 1B model was fitted into the 1BP density using a rigid-body fit in ChimeraX51 (v.1.10.1), followed by a jiggle-fit and all-atom refinement in Coot49 (v.0.9.8.96). Position 53 was mutated into a threonine and the model was extended to five β-rungs, which were refined together in phenix.real_space_refine (PHENIX v.1.21.2). After refinement, the outer two β-rungs were removed in Coot. NCS and secondary structural restraints were included during the iterative refinement process.
FLIM analysis of aSyn inclusion pathology
h-FTAA was synthesized and purified as previously described36 and paraffin-embedded sections of 1B-injected WT mouse brains (129SV) and regions from human donor brain samples were stained with h-FTAA and processed for immunofluorescence against pS129aSyn (EP1536Y) for the inclusions or against human aSyn (LB509) for the 1B inoculate and counterstained with the nuclear marker DAPI as previously described37. To confirm previous observations regarding the ability of h-FTAA to discriminate between Lewy bodies and GCIs using FLIM37, human brain samples from one individual with MSA and one individual with DLB were obtained from the repository of the University Hospital of Bordeaux (CRB-BBS). Participants or a legal representative had given informed written consent for collecting and using clinical and post-mortem data, and mandatory regulatory approval for post-mortem brain tissue use was obtained from the French Ministry of Higher Education and Research (MESR AC-2024-6715, DC-2024-6714). FLIM was performed on the Leica SP8 WLL2 confocal microscope on an inverted stand DMI6000 (Leica Microsystems), using the HCX Plan Apo CS2 ×63 oil NA 1.40 objective. This microscope was equipped with a pulsed white-light laser 2 (WLL2) with freely tuneable excitation from 470 to 670 nm (1 nm steps) and a diode laser at 405 nm. A FALCON module enabled us to perform FLIM measurement based on the time correlated single photon counting technique. The FLIM analysis was done using phasor plot representation of the data and was focused on pS129aSyn-positive inclusions37 or LB509-positive regions in the case of the 1B inoculate. Similitude analysis was performed using the Jaccard index52. For Gallyas silver impregnation, mouse brain sections were processed as previously described38 using a commercial Gallyas staining kit (Morphisto).
AFM analysis
1B aSyn fibrils were deposited onto a freshly cleaved mica disk and left to adhere for 15 min in a humid chamber at room temperature. The sample was rinsed and imaged in saline buffer solution (H2O, HEPES 20 mM, NaCl 100 mM, pH 7.4). Atomic force microscopy (AFM) imaging was performed using the Dimension FastScan setup (Bruker) operating in PeakForce Quantitative Nano-Mechanics (PF-QNM) mode in liquid at room temperature, using sharp SNL-C probes (Bruker, Silicon tips on silicon nitride cantilevers), with a nominal spring constant of 0.24 N m−1, a resonance frequency of 56 kHz and a nominal tip radius of 2 nm. Fibrils were imaged with a peak force frequency of 1 kHz, a scan rate of 0.5 Hz and a constant setpoint force of 500 pN. Image analysis was performed using Nanoscope analysis (Bruker).
Statistics and reproducibility
For all experiments/conditions without quantitative analyses, the images shown were representative of at least three independent observations. In all other cases, statistical analyses data were presented as means ± s.d. or as otherwise stated in the figure legends, and were performed using GraphPad Prism (v.9). The numbers of independent samples as well as the statistics tests used are indicated in the figure legends for each experiment. No statistical methods were used to predetermine sample size. Sample size was determined based on our previous publications. Data distribution was assumed to be normal, although this was not tested. Data assignment, organization and collection were randomized. Data collection and analysis were not performed blindly. No animals or datapoints were excluded from the analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.