Animals
ThCre mice (B6.Cg-7630403G23RikTg(Th-cre)1Tmd/J, stock no. 008601) and Cx3cr1GFP/+ mice (Cx3cr1tm1Litt/LittJ, stock no. 008451) were purchased from the Jackson Lab. Npyflox/flox mice were a donation from the I. Kalajzic Laboratory at the Department of Reconstructive Science, University of Connecticut44 under the material transfer agreement (MTA). Tissues of NPY-GFP mice (B6.FVB-Tg(Npy-hrGFP)1Lowl/J) were sourced from the Tamas Horvath Laboratory at Brandy Memorial Laboratory, Yale University. Tissues from Npy1rCre;Rosa26tdTomato mice were provided by M. Roberts at the Department of OtolaryngologyâHead and Neck Surgery, University of Michigan. Sympathetic neuron-specific NPY-cKO mice were generated by crossing ThCre mice with Npyflox/flox mice. Diet-induced obesity was achieved by feeding mice an HFD (Diet Research, product no. D12492) when they were 7âweeks old, and this feeding regime lasted for 10âweeks. The body weight of each mouse and food consumption in each cage were recorded weekly. All mice were group housed in standard housing under controlled room temperature (21â23â°C) and 50% humidity under a 12/12âh light/dark cycle and given access to diet and water ad libitum. All experimental procedures were performed on living animals in accordance with the UK ANIMALS ACTS 1986 under the project licence (PPL no. P80EDA9F7) and personal licences granted by the UK Home Office. Ethical approval was provided by the Ethical Review Panel at the University of Oxford.
Energy expenditure and measurement of respiratory exchange rate
Animals aged 6âweeks were analysed for oxygen consumption, carbon dioxide production, energy expenditure and respiratory exchange rate using an indirect calorimetry system (Panlab, Harvard Apparatus, LE405 Gas Analyzer and Air Supply & Switching). Animals were maintained in individual cages following a 12/12âh light/dark cycle with water and food supplied ad libitum, and under controlled room temperature (21â23â°C) and 50% humidity. Metabolic data were collected for 5âdays following a 2âday acclimatation period. Animalsâ body weight (g) and food (g) were measured before entering and after exiting the cage. Results were normalized by weight, and graphs and statistical analysis were obtained using the CalR Web-based Analysis Tool for Indirect Calorimetry Experiments (v.1.3)45.
Locomotor activity and measurement of body composition
Animals aged 8â9âweeks were analysed for spontaneous locomotor activity (Panlab, Harvard Apparatus, LE001âPH Multitake Cage). Animals were maintained in individual cages following a 12/12âh light/dark cycle with water and food supplied ad libitum, and under controlled room temperature (21â23â°C) and humidity. Data were collected for 72âh following a 1âday acclimatation period. Activity was recorded using COMPULSE v.1.0 software (Panlab). Animals aged 7â9âweeks were analysed for body composition using a Minispec LF50 (Brucker).
Fasting and thermoimaging
Animals aged 12â14âweeks were used for thermoimaging. The data shown in Fig. 5e were recorded using an Optris thermocamera (Optris PIâ160 with a standard 61âlens), and the data in Extended Data Fig. 10l were recorded using a FOTRIC 225âs infrared camera and analysed with FOTRIC software (v.5.0.8.214). Animals were single housed and placed under the thermocameras, with their back shaved to expose the skin above iBAT. Animals were acclimatized for 4âdays in individual cages with ad libitum access to food and water at room temperature under a 12/12âh light/dark cycle before a 14-h fast. Mice had ad libitum access to water during fasting.
iBAT temperature was recorded every 1âs over a 6-day period by storing the temperature of the warmest pixel in view using the software provided by the camera manufacturer (rel. 2.0.6, Optris PIX Connect). iBAT temperature at 0 and 14âh was taken as an average of temperatures during a 1-h period.
Cold-exposure experiment
Animals aged 20âweeks were single housed in a thermoneutral environment (31â°C, 50% humidity) under a 12/12âlight/dark cycle for 1âweek, with ad libitum access to food and water. Mice were then cold-challenged at 4â°C and their rectal temperature measured every 1âh using a rectal probe. Animals were killed immediately following 8âh of cold exposure and their iBAT harvested for qPCR with reverse transcription analysis.
Stereotaxic injection
For viral injection, mice were anaesthetized with avertin (240âmgâkgâ1 intraperitoneally) and fixed on a stereotaxic holder (RWD, Life Science). Either AAV2/9-hSyn-Cre-EGFP-WPRE-pA (Taitool, catalogue no. S0230-9, 2âÃâ1,012âviral genomes mlâ1, 200ânl per side) or AAV2/9-hSyn-EGFP-WPRE-pA (catalogue no. S0237-9, Taitool, 2âÃâ1,012âviral genomes mlâ1, 200ânl per side) was bilaterally injected into the nucleus tractus solitarius (NTS) (NTS coordinates anteroposterior, mediolateral, dorsoventral â7.5, ±0.35 and â4.75âmm, respectively) of Npyflox/flox mice.
Immunofluorescent staining
Superior cervical ganglia and sympathetic axon bundles were dissected from adipose tissues of mice perfused with 20âml of PBS, and the tissues were fixed in 4% paraformaldehyde (PFA, 043368.9âM, ThermoFisher) for at least 4âh. Cells collected following in vitro experiments were washed once with PBS and fixed using 4% PFA for at least 4âh. Axon bundles were first stained by incubation overnight at 4â°C with primary antibodies dissolved in the permeabilizing buffer (3% bovine serum albumin, 2% goat serum, 0.1% TritonâX-100 and 0.1% sodium azide in PBS), followed by incubation with secondary antibodies and DAPI dissolved in the permeabilizing buffer for 1âh with gentle agitation. As for immunofluorescence in fixed cells, cells were first incubated with the permeabilizing buffer for 1âh at room temperature and then stained overnight at 4â°C with primary antibodies diluted in the permeabilizing buffer. Samples were then washed and stained with DAPI and secondary antibodies for 1âh at room temperature. Sympathetic fibres or fixed cells were then mounted on slides with Fluoromount-G mounting medium (ThermoFisher, catalogue no. 00-4958-02).
For cryosection, samples were first embedded in OCT (VWR, catalogue no. 361603E) and frozen at â20â°C until cryosectioning. Samples were cut into 10-µm-thick sections and mounted on charged SuperFrost Plus slides (VWR, catalogue no. 631-0108), followed by staining using the same procedure as for fixed cells.
For paraffin embedding, fixed samples were processed in 70, 80 and 90% ethanol and then in 100% ethanol, 100% xylene and 60â°C paraffin wax, three times for each condition. Samples were embedded in paraffin wax, sectioned at 7âµm, mounted on coverslips and dried in an oven at 45â50â°C overnight. Before staining, samples were deparaffinized twice in 100% xylene for 5âmin, rehydrated twice in 100% ethanol and then once each in 95 and 80% ethanol and water for 10âmin. Antigen retrieval was done by the addition of 0.05% trypsin to each slide and incubation at 37â°C for 20âmin. After washing with running water, samples were stained using the same procedure as for fixed cells.
For staining, whole-mount, PFA-fixed ganglia were dehydrated once in each of 30, 50, 70 and 90% ethanol, twice in 100% ethanolâwith shaking for 20âmin at each concentrationâand then rehydrated in 90, 70, 50 and 30% ethanol. Ganglia were then digested with 1.5âUâmlâ1 dispase-1 (Roche, catalogue no. 04942078001), 0.5âmgâmlâ1 collagenase (Sigma, catalogue no. C2674) and 300âμgâmlâ1 hyaluronidase (Sigma, catalogue no. H3884) diluted in PBS, with shaking at 37â°C in a water bath for 30âmin. Ethanol-treated ganglia were blocked in blocking solution (3% bovine serum albumin, 2% goat serum, 0.1% Triton X-100 and 0.1% sodium azide in PBS) for 2âh and then incubated with primary antibodies diluted in the permeabilizing buffer for 3âdays at 4â°C. Three days later, ganglia were incubated in secondary antibodies diluted in the permeabilizing buffer for a further 3âdays at 4â°C. Finally, ganglia were dehydrated once each in 30, 50, 70 and 90% ethanol and twice in 100% ethanol then cleared using ethyl cinnamate (ECi). Cleared ganglia were mounted in a coverslip slide sandwich filled with ECi.
Images were acquired using a Zeiss LSM880 confocal microscope with Zen-black software (v.2.1). Samples were imaged using either (1) a Ã10/0.45 numerical aperture (NA) objective with a voxel size of 1.04âμm (x), 1.04âμm (y) and 3.64âμm (z), (2) a Ã20/0.8âNA objective with voxel size of 0.52âμm (x), 0.52âμm (y) and 1.00âμm (z) or (3) a Ã63/1.4âNA oil-immersion objective with a voxel size of 0.13âμm (x), 0.13âμm (y) and 1.00âμm (z). Solid-state lasers of 405, 561 and 633ânm and an Argon 488 laser were used for DAPI, AF488, AF546 and AF647 fluorophores, respectively.
Clearing of adipose tissue
Whole fat pads were stained and cleared using a modified version of iDISCO as previously described18,46. In brief, WAT or BAT was dissected from mice perfused with 20âml of PBS, and the tissues were fixed with 4% PFA overnight. Tissues were pretreated once each with 20, 40, 60 and 80% ethanol and twice in 100% methanol for 30âmin, all at room temperature, and then bleached with 5% H2O2 diluted in 100% methanol for 24âh at 4â°C with shaking. Bleached samples were rehydrated using 80, 60, 40 and 20% methanol for 30âmin each and then in PTwH buffer (0.2% Tween-20, 10âμgâmlâ1 heparin and 0.02% sodium azide in PBS) for 30âmin. Afterwards, samples were incubated overnight in permeabilizing solution (20% DMSO, 0.2% Triton X-100, 0.3âM glycine in PBS) at 37â°C in a water bath and blocked using blocking buffer (10% DMSO, 2% Triton X-100, 0.02% sodium azide and 5% goat serum in PBS). For immunolabelling, samples were incubated for 5âdays, with shaking, in primary antibodies diluted in antibody dilution buffer (5% DMSO, 5% goat serum, 0.2% Tween-20 and 10âμgâmlâ1 heparin in PBS) at 37â°C, washed with PTwH for 1âday and then incubated for a further 3âdays with secondary antibodies and DAPI diluted using antibody dilution buffer at 37â°C, with shaking. Immunolabelled tissues were embedded in 1% agarose and dehydrated in 20, 40, 60, 80 and 100% methanol for 1âh each, and then in 100% methanol overnight at room temperature. Samples were incubated in dichloromethane (Sigma, catalogue no. 270997) until they sank and were then incubated in dibenzyl ether (Sigma, catalogue no. 179272) until clear. Samples were stored in dibenzyl ether at room temperature and transferred into ECi before imaging. Images of cleared tissues were acquired using a Miltenyi Biotec UltramicroscopeâII light-sheet microscope; step size was 8âμm, thickness of the light sheet was 3.98âμm, horizontal dynamic focusing was set to eight steps and exposure time was 180âms. Samples were illuminated using a bidirectional light sheet and scanned under a Ã2/0.5âNA objective with voxel size 4.03âμm (x), 4.03âμm (y) and 8âμm (z). A 488-nm laser with a 525/50 filter, a 561-nm laser with a 620/60 filter and a 638-nm laser with a 680/30 filter were used for AF488, AF546 and AF647, respectively.
Dextran-70kDa injection and intravital microscopy
Animals were anaesthetized with ketamine (100âmgâkgâ1) and xylazine (10âmgâkgâ1) and a small incision was made to expose epididymal white adipose tissue for microscopy analysis. Intravital images of space resolution 1,024âÃâ1,024 pixels from epididymal WAT were obtained simultaneously using an optical microscope with coherent anti-Stokes Raman scattering, two-photon excitation fluorescence and a bright-field microscope, with a confocal LSM 780-NLO Zeiss inverted microscope Axio Observer Z.1 (Carl Zeiss). All procedures were performed at the National Institute of Science and Technology on Photonics Applied to Cell Biology at the State University of Campinas. Adipocyte images from coherent anti-Stokes Raman scattering were acquired using two lines of lasers in wavelengths λpumpâ=â803ânm and λStokesâ=â1,040ânm, and fluorescence images were acquired by two-photon excitation fluorescence using the exogenous fluorescence dye tetramethylrhodamine isothiocyanate dextran (Sigma, catalogue no. T1162) in wavelength of excitation λStokesâ=â1,040ânm. Following identification of blood vessels and acquisition of an initial image the video was started and, after ten frames, 50âµl (30âmgâkgâ1) of Dextran-70KDa (Sigma, catalogue no. T1162) was injected into the orbital plexus of the mouse. The video continued recording for approximately 12âmin to capture vessel fluorescence and leakage. Fluorescence intensity over time was analysed for the whole duration of the video. A static image was analysed following dextran administration using Fiji47. For evaluation of tissue leakage, a region of interest was selected inside the vessel and another in the tissue (outside the vessel). Fluorescence intensity was measured in both areas, and the intensity ratio between the tissue and within the vessel was calculated. Blood flow was calculated using the average of the raw intensity of dextran inside the artery over 100âs immediately following stabilization of the dextran signal after administration.
Antibodies
The following primary antibodies were used for immunofluorescent staining: rat anti-CD31 (BioLegend, catalogue no. 102501, MEC13.3, 1:100 dilution), rat anti-PLVAP (BioLegend, catalogue no. 120503, MECA32, 1:100 dilution), rabbit anti-DES (abcam, catalogue no. ab15200, 1:500 dilution), rabbit anti-NPY (Cell Signaling, catalogue no. D7Y5A, 1:500 dilution), rabbit anti-NPY (abcam, catalogue no. ab30914, 1:500 dilution), chicken anti-TH (Aves Labs, catalogue no. TYH73787982, 1:500 dilution), rabbit anti-TH (Sigma, catalogue no. Ab152, 1:500 dilution), mouse anti-NPY1R (Santa Cruz, catalogue no. sc-393192, 1:200 dilution), rat anti-PDGFRα (BioLegend, catalogue no. 135902, APA5, 1:200 dilution), rabbit anti-TAGLN (abcam, catalogue no. ab14106, 1:250 dilution), Cy3 anti-αSMA (Sigma, catalogue no. C6198, 1A4, 1:250 dilution), goat anti-SOX17 (R&D, catalogue no. AF1924, 1:250 dilution), goat anti-EPHB4 (R&D, catalogue no. AF3034, 1:250 dilution) and rabbit anti-UCP1 (abcam, catalogue no. ab10983, 1:500 dilution).
The following antibodies were used for fluorescent activated cell sorting (FACS) and flow cytometry: AF700 anti-CD45 (BioLegend, catalogue no. 103128), BUV395 anti-CD45 (BD Horizon, catalogue no. 564279), Pacific Blue anti-CD31 (BioLegend, catalogue no. 102421), APC anti-PDGFRa (BioLegend, catalogue no. 135907), AF488 anti-NG2 (Sigma, catalogue no. MAB5384A4) and AF488 anti-DES (abcam, catalogue no. ab185033, Y66). All antibodies for FACS and flow cytometry were diluted at 1:500. The LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (1:1,000, ThermoFisher, catalogue no. L10119) was used for live/dead staining.
qPCR
RNA extraction was performed using Trizol reagent (ThermoFisher, catalogue no. 15596026), complementary DNA was synthesized using SuperScriptâII Reverse Transcriptase (Invitrogen, catalogue no. 18064022) and qPCR was performed using Power SYBR Green PCR Master Mix (LifeTech, catalogue no. 4368706). Data were recorded using a StepOne qPCR system and analysed with StepOne Software v.2.3. Primers used in this research are listed in Supplementary Table 1.
The ÎCt method was used to quantify gene expression using the following formula: relative expressionâ= 2^â(â(Cttarget geneâââCtreference gene)). The ÎÎCt method was used to compare the thermogenic and adipogenic gene expression shown in Fig. 5b and Extended Data Figs. 5b,d,g, 9l,m and 10m.
The qPCR data shown in Extended Data Fig. 10m were collected using the CFX96 Real-Time PCR Detection System (Bio-Rad) with TB Green Premix Ex TaqâII (TaKaRa, catalogue no. RR820A).
Protein extraction and blood plasma isolation for NPY ELISA
Proteins were extracted from iWAT as previously described48. In brief, dissected iWAT was placed in homogenizing tubes filled with 300âμlâ50âmgâ1 RIPA lysis buffer (Sigma, catalogue no. 20188) containing 50âμM DPP-IV inhibitor (Sigma, catalogue no. DPP4-010) and 500,000âIUâmlâ1 aprotinin (Sigma, catalogue no. A6103-1MG) and homogenized using a Precellysâ24 homogenizer. Lipid in the tissue homogenize was removed by centrifuging at 20,000Ãârcf at 4â°C for 15âmin, and the clear portion retained. The process was repeated three times to ensure complete removal of lipid.
For terminal blood collection, mice were euthanized by intraperitoneal injection of 10âμlâgâ1 pentobarbital and blood was then collected from the left ventricle using a 25âG needle and a syringe coated with 100âmM EDTA. Blood was then placed in tubes with 5âμl of 100âmM EDTA and centrifuged at 1,000Ãârcf at 4â°C for 15âmin to separate plasma from blood cells, and then the supernatant was transferred to fresh tubes with DPP-IV inhibitor (final concentration 50âμM) and aprotinin (final concentration 500,000âIUâmlâ1). The concentration of NPY in mouse iWAT and blood plasma was determined using an NPY ELISA kit (Merck, catalogue no. EZRMNPY-27K). ELISA data were recorded using a FLUOstar Omega microplate reader with Omega v.6.20 software.
Single-cell suspension and flow cytometry
Dissected adipose tissues were minced and digested in an enzyme mixture (for each sample, 500âμl of collagenaseâII (4âmgâmlâ1, Sigma, catalogue no. C6885), 500âμl of hyaluronidase (5.3âmgâmlâ1, equivalent to 40,000âUâmlâ1, Sigma, catalogue no. H3884) and 5âμl of DnaseâI (BioLabs, catalogue no. M0303L)) in a 37â°C water bath, with shaking, for 45âmin, and samples pipetted every 10âmin. Digestion was stopped by the addition of FACS buffer (PBS containing 2% fetal bovine serum (FBS)), and single-cell suspensions were collected by filtering the digested sample using EASYStrainer cell sieves (70âμm mesh; Greiner, catalogue no. 542070).
For preparation of samples for sorting or flow cytometry, cells were first treated with red blood cell lysis buffer (BioLegend, catalogue no. 420301) to remove red blood cells and then treated with Fc block (ThermoFisher, catalogue no. 14-9161-73) before staining with antibodies. Before immunolabelling for intracellular markers, cells were fixed and permeabilized using the eBioscience Intracellular Fixation and Permeabilization Buffer Set (ThermoFisher, catalogue no. 88-8824-00). Cell sorting was performed using a BD FACSAriaâIII sorter, and flow cytometry data were acquired with either a BD FACSAriaâIII sorter or a BD LSRFortessa X20 cytometer with BD FACSDiva v.6.0 software. Cytometry data were analysed using FlowJo v.10.8.1.
Isolation and primary culture of mural cells and in vitro stimulation
Mural cells were isolated and cultured following a modified version of the protocol previously published49. In brief, mice were euthanized intraperitoneally with 10âμlâgâ1 pentobarbital and perfused with 15âml of PBS to remove blood. The iBAT SVF was isolated using the method described above. Cells were then seeded in 10âcm dishes with 10âml of DMEM with 10% FBS for 2âdays for the cells to attach to the bottom. Afterwards, 5âml of the medium was replaced with mural cell-specific medium (DMEM with 2% FBS, supplied with 1% mural cell growth supplement (Science Cell, catalogue no. 1252)). Every other day, 5âml of medium in dishes was refreshed until the cells grew confluent. For acquisition of pure mural cells, cells were labelled with an APC anti-CD104b antibody (1:100, BioLegend, catalogue no. 136007) and mural cells were sorted using the MojoSort magnetic sorting protocol with MojoSort buffer (BioLegend, catalogue no. 480017) and anti-APC nanobeads (BioLegend, catalogue no. 480071).
To perform in vitro coculturing experiments, cells were seeded at 5âÃâ104 per millilitre on glass coverslips and the indicated concentrations of PDGF-BB (R&B, catalogue no. 220-BB) and NPY (Cayman Chemical, catalogue no. CAY15071) were added to cells 24âh following cell seeding. Cells were collected 5âdays later and either fixed with 4% PFA for imaging or lysed with Trizol for RNA extraction and qPCR.
Cell lines
The RAW264.7 mouse macrophage cell line (Sigma, catalogue no. 91062702) was purchased without further authentication or testing for mycoplasma contamination. The complete medium for cell culture includes high-glucose DMEM with glutamate (Sigma, catalogue no. 41965039) and 10% FBS (Sigma, catalogue no. 12133âC).
The 3T3-L1 preadipocyte cell line was a gift from R. Klemm at the Department of Physiology, Anatomy and Genetics, University of Oxford, and used without further authentication or testing for mycoplasma contamination. The complete medium for cell culture includes high-glucose DMEM with glutamate (ThermoFisher, catalogue no. 41965039) and 10% calf serum (Sigma, catalogue no. 12133âC). For subculture of preadipocytes the medium was transferred, and cells were washed with 1âml of prewarmed 0.05% trypsin (ThermoFisher, catalogue no. 25300054) and digested with 200âμl of trypsin at 37â°C for 5â10âmin. Afterwards, digestion was stopped and cells resuspended using the complete medium.
Induction to white and thermogenic adipocytes
For differentiation of 3T3-L1 cells to white adipocytes, cells were seeded in 12-well plates at a density of 1âÃâ105 per millilitre. The medium was refreshed until cells were confluent. Two days following cell confluence, induction medium (10% FBS, 500âμM 3-isobutyl-methylxanthine and 1âμM dexamethasone in DMEM) with or without 1âμgâmlâ1 insulin was added to each well; 3âdays later the induction medium was replaced with maintenance medium (DMEM with 10% FBS with or without 1âμgâmlâ1 insulin). Subsequently the medium was refreshed with maintenance medium every 2âdays. Total differentiation time for each well was 8âdays. NPY treatments in experimental groups started when the induction medium was added and lasted throughout the whole differentiation process.
SVF and mural cells were differentiated to thermogenic adipocytes as previously reported6,30. SVF and mural cells were isolated and seeded in 12-well plates at a density of 1âÃâ105 per millilitre. When cells reached 95% confluency, beige cell induction medium (DMEM with 10% FBS, 125âμM indomethacin, 5âμM dexamethasone, 500âμM 3-isobutyl-methylxanthine and 0.5âμM rosiglitazone) was added to each well; 2âdays later the induction medium was replaced with maintenance medium (DMEM with 10% FBS, 5âμgâmlâ1 insulin and 1ânM triiodothyronine). Maintenance medium was refreshed every 2âdays. Total differentiation time for each well was 8âdays. NPY treatments in the experimental groups started following the addition of induction medium and lasted throughout the whole differentiation process. Oxygen consumption was measured using a Seahorse XF Analyzer.
Cell proliferation assay
The cell proliferation assay was performed with the Click-iT EdU Cell Proliferation Kit (ThermoFisher, catalogue no. C10340) for imaging. Mural cells were seeded at 0.5âÃâ105 per millilitre in 12-well plates on coverslips and cultured overnight before experiments. Cells were then labelled with EdU and cultured with or without 1âμM NPY or 2âμM PD98059 (Cell Signaling, catalogue no. 9900âS) for 6âh in medium for mural cells (low-glucose DMEM with 2% FBS). Cells were then fixed with 4% PFA and permeabilizing buffer for 30âmin each, and EdU was labelled with AF647 using the Click-iT Plus reaction cocktail. Following immunofluorescent staining with anti-DES, cells were imaged using a Zeiss LSM880 confocal microscope with Zen-black software (v.2.1), with a Ã20/0.8âNA objective and voxel size of 0.52âμm (x), 0.52âμm (y) and 1.00âμm (z). An area of 3,072âÃâ3,072 pixels was imaged for each biological replicate.
scRNA-seq dataset analysis
Public scRNA-seq datasets were downloaded from Gene Expression Omnibus (GEO) and analysed using the following method. Cells with fewer than 200âunique detected genes or over 5% mitochondrial counts were discarded. After filtering, the geneâx cell matrix was normalized using âNormalizeData()â in Seurat v.4.2.0 (ref. 50) in R (v.4.2.2). Data were then scaled using âScaleData()â, and linear dimensional reduction performed by principal component analysis and calculation of UMAP coordinates for all cells using Seurat v.4.2.0. Cells were clustered using âFindNeighbour()â, with dimensions set to 15, and âFindClusters()â, with resolution set to 0.5. Each cluster was identified based on differentially expressed genes and known markers in the published literature5,22,29,51,52,53.
Figure quantification
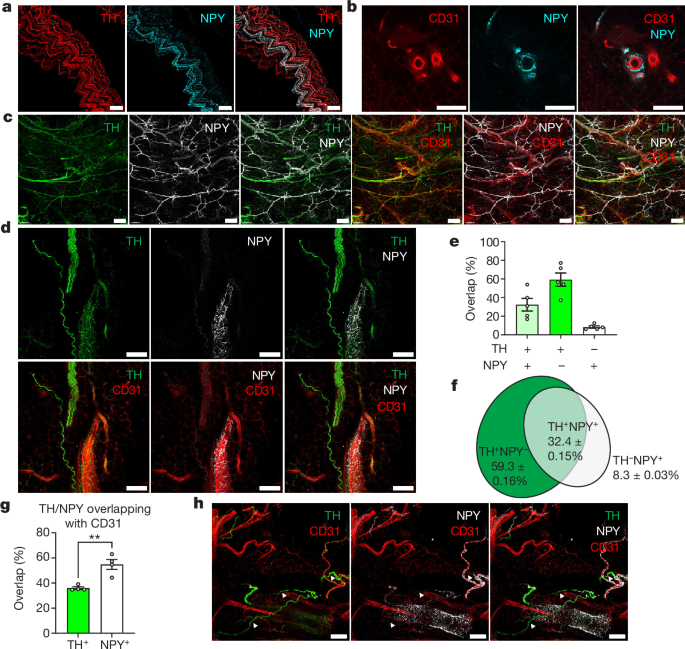
Overlapping between TH, NPY and CD31 was calculated using JACoP54, a Fiji plug-in47. To make this calculation, 472.33, 472.33 and 30âμm xyz regions were randomly picked and maximally projected to z using Fiji script. Labelled areas were automatically segmented using a threshold set by default, and overlapping percentages were calculated automatically by JACoP.
Innervation of NPY+ axons was quantified using the âSurfaceâ tool in Imaris v.9.2. Labelled areas of NPY+ axons and CD31+ vessels in whole cleared iWAT were automatically segmented, and the innervation of NPY in vasculature was calculated as volumeNPY+/volumeCD31+. The coverage of DES+ mural cells in iWAT was calculated similarly, as volumeDES+/volumeCD31+. Confocal images showing NPY+ innervation were quantified using Fiji with an automatic unbiased method. AreaNPY+ and areaCD31+ were segmented using the Otsu thresholding method and measured with the âmeasureâ program.
The percentage of EdU+ cells was counted with an unbiased automatic method using âdetect particlesâ in Fiji47. Threshold was set automatically using the Otsu thresholding method.
The percentages of PDGFRα+ and DES+ cells were counted manually based on PDGFRα and DES signals. The percentages of TH+ and NPY+ neurons in the hypothalamus and ventral tegmental area were also counted manually. To calculate ganglionic mural cell coverage, 531, 531 and 30âμm xyz views were randomly picked and maximally projected to z using Fiji script. Labelled areas were segmented using a threshold set by default, and coverage was calculated as area NPY+/area CD31+.
The size of adipocytes in WAT and iBAT was quantified as previously described55 using Fiji. In brief, WAT and iBAT were processed to paraffin-embedded sections (3âμm), stained with haematoxylin and eosin and scanned into digital images using a NanoZoomer-SQ Digital slide scanner (Hamamatsu). Images were randomly picked and the plug-in Analyse Particles was used to count the number and measure the size of adipocytes and droplets in iBAT. For each iBAT, 11,000âlipid droplets were measured.
Statistics and reproducibility
GraphPad Prism v.9.5.0 was used for statistical analysis. Mean and s.e.m. were used to represent a sample. Chi-squared and paired two-tailed Studentâs t-tests were used to compare two samples; analysis of variance was used to compare multiple data points. No statistical methods were used to predetermine sample sizes.
All experiments were replicated at least twice with the same conclusion, and all in vivo experiments were replicated in at least two independent cohorts. The exact numbers of replicated experiments are stated in each figure legend. Samples and animals were randomly allocated to experimental groups and proceeded in experiments, and data were collected and analysed blind and ad hoc registered to groups or genotypes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.