Sample preparation
Rabbit skeletal actin in monomeric form (globular actin (G-actin)) was prepared as previously described39. Polymerization to F-actin was performed by mixing about 300 µM G-actin with 10% (v/v) cation exchange buffer (3 mM MgCl2, 11 mM EGTA, pH 7.0), incubating for 5 min on ice, adding 10% (v/v) polymerization buffer (120 mM MOPS, 300 mM KCl, 12 mM MgCl2, 1 mM EGTA, pH 7.0) and incubating the mixture overnight on ice. Mouse myosin-5a head fragment (subfragment 1, S1), coding for amino acids 1–797 (one IQ calmodulin-binding motif) and carrying the switch 1 S217A substitution, loop 2 DDEK(594–597) deletion and C-terminal Flag purification tag (Supplementary Fig. 2), was expressed using pVL1392 baculovirus transfer vector and purified as previously described5. Disodium ATP was obtained from Roche, and ADP was obtained from Sigma Aldrich.
Kinetic measurements
Transient kinetics of actomyosin ATP hydrolysis were measured by use of a Hitech Scientific stopped-flow apparatus with single or double mixing, as appropriate. All stopped-flow experiments were carried out at 20 °C with a final buffer concentration of 37.5 mM potassium acetate, 25 mM KCl, 10 mM MOPS (pH 7.0), 2.25 mM MgCl2, 0.1 mM EGTA, 0.25 mM dithiothreitol in the cell. See Supplementary Fig. 3 for specific method information.
Time-resolved cryo-EM grid preparation
Time-resolved cryo-EM experiments were carried out using a custom-built set-up previously described21 with modifications to allow two mixing steps. A photo and schematic of the set-up are shown in Supplementary Fig. 1. The flow rates for each individual syringe were 2.1 µl s−1. In the first mixing step, myosin-5 at 51 µM in 10 mM MOPS, 100 mM KCl, 3 mM MgCl2, 0.1 mM EGTA pH 7.0 was mixed 1:1 with 1 mM ATP in reaction buffer (10 mM MOPS, 50 mM potassium acetate, 2 mM MgCl2, 0.1 mM EGTA pH 7.0). The mixture of myosin and nucleotide at a flow rate of 4.2 µl s−1 was met by two 2.1 µl s−1 flows of F-actin at 25 µM (subunit concentration in reaction buffer) in the flow-focusing region of the spray nozzle to create a mixture of actin and myosin comprising 13 µM myosin, 13 µM actin, 250 µM ATP, 10 mM MOPS, 38 mM potassium acetate, 25 mM KCl, 2 mM MgCl2 and 0.1 mM EGTA at pH 7.0, and a total flow rate of 8.4 µl s−1. This final mixture was sprayed onto an EM grid.
The average time delay from the first mixing step to the spray nozzle was 2.2 s, given a flow rate of 4.2 µl s−1, tube length of 7 cm, inner diameter of 0.38 mm and dead volumes of 1.0 and 0.3 µl for mixer and nozzle, respectively. The spray nozzles used here have been described and characterized previously4,40. The nozzle-to-grid distance at the point of sample application was 1.3 cm, and the droplet speed was ≥30 m s−1, resulting in a time-of-flight for the droplets of less than 1 ms. With a vertical distance of 1.7 cm between the spray nozzle and the liquid ethane surface and a grid speed of 1.8 m s−1, the time delay was calculated to be 10 ms (10 ± 2 ms). The nozzle was operated in spraying mode with a spray gas pressure of 2 bar.
A longer time delay of about 120 ms was obtained by increasing the vertical distance between the nozzle and the ethane surface to 5.2 cm and pausing the grid after passing the spray. In these experiments, the sample mixture was incubated for an additional ≈100 ms on-grid, before plunging it into liquid ethane for vitrification. The total time delay from droplet application to vitrification was 120 ms (122 ± 5 ms), including deceleration, 100 ms pause and acceleration. Otherwise, the conditions for grid preparation were the same as for the 10 ms time point.
All grids were prepared at room temperature (about 20 °C) and at >60% relative humidity in the environmental chamber of the time-resolved EM device. Self-wicking grids were supplied by SPT Labtech and used after glow discharge in a Cressington 208 Carbon coater with a glow-discharge unit for 60 s at 0.1 mbar air pressure and 10 mA. Four replicate grids were prepared for each time point, three of which were taken forward for data collection.
A typical feature of grids made by spraying is thicker ice compared to those prepared using more standard approaches41, such as by the use of the Vitrobot. This is due to the requirement for the drops to ‘thin’ after deposition on the grid, which can be limited by the short time between spraying and vitrification. This increase in ice thickness will be an important factor in limiting the resolution to about 4.0 Å, alongside conformational flexibility18.
Rigor actomyosin cryo-EM grid preparation
A 3 µl volume of pre-mixed F-actin and myosin (1:1 ratio with a final concentration of 1 µM) was applied to a Quantifoil R2/2 300 mesh grid that had been glow-discharged in a Cressington 208 Carbon coater for 30 s at 0.1 mbar air pressure and 10 mA before use. Grids were prepared by use of a Vitrobot Mark IV (ThermoFisher), with a blot time of 6 s and a blot force of 6 at 4 °C and 95% humidity with vitrification in liquid ethane.
EM data gathering and processing
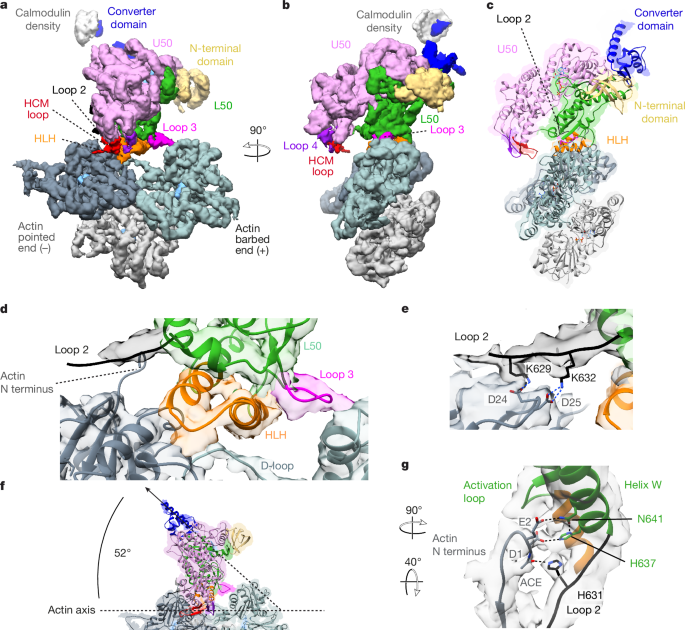
EM data were collected on a Titan Krios microscope equipped with a Gatan K2 direct electron detector operated in counting mode using the EPU software version 2.12 distributed by Thermo Scientific. The main data collection and processing parameters for the time-resolved EM grids are listed in Extended Data Table 1. A schematic overview of the processing pipeline is given in Supplementary Fig. 4. Data from three grids were collected for each time point. All processing was carried out using RELION-3.1 (ref. 42), unless otherwise mentioned. Micrographs were corrected for beam-induced motion using MotionCor2, and CTF estimation was carried out using GCTF43,44. Actin filaments were manually picked and processed using standard helical processing methods45 (Supplementary Fig. 4). After CTF-refinement and Bayesian polishing, all six datasets were combined, and a helical consensus structure was calculated (Supplementary Fig. 4c). Using focused three-dimensional (3D) classification without alignment (non-helical) and a mask that covered the central myosin-binding site (Supplementary Fig. 4d), particles were classified into actomyosin states or bare actin, with a small fraction of particles left unassigned. Despite performing multiple rounds of 3D classification, we found that actomyosin particles were classified into either a primed actomyosin state, with a primed lever and an open actin-binding cleft, or a postPS actomyosin state, with a postPS lever and a closed actin-binding cleft (Supplementary Fig. 4d,e). No other actomyosin states were identified. The final reconstruction of free actin was obtained by helical refinement. Primed and postPS actomyosin were refined helically and after partial signal subtraction, as single particles (Supplementary Fig. 4c–e). Post-processing was performed in RELION and in DeepEMhancer46.
To quantify the conversion of primed actomyosin to postPS actomyosin between the 10 ms and 120 ms time points, and consistency of this between grids, each particle used in the primed and postPS structures was assigned back to the grid from which it was imaged, and then the total number of primed particles was expressed as a proportion of all particles assigned to each grid.
For unbound myosin-5, the processing parameters are listed in Extended Data Table 1, with an overview of the processing pipeline shown in Supplementary Fig. 5. Unbound myosin-5 particles were picked from a subset of micrographs of the 120 ms time-resolved data. As a result of thicker ice, unbound myosin particles were not picked from the 10-ms data. After one round of 2D classification, good particles were used to train a crYOLO model47. With the trained model, particles were picked from the entire 120-ms dataset, leading to a final selection of 23,930 particles after 1 round of 2D and 1 round of 3D classification. The final 3D refinement after Bayesian polishing was performed using non-uniform refinement in cryoSPARC48.
For rigor actomyosin-5, the processing parameters are listed in Extended Data Table 3, with an overview of the processing pipeline shown in Supplementary Fig. 6. Particles were manually picked from a subset of micrographs, and after one round of 2D classification, good particles were used to train a crYOLO model47. With the trained model, particles were picked from the entire rigor dataset, leading to a final selection of 85,986 particles after 1 round of 2D classification. The particles then underwent two rounds of 3D helical refinement and Bayesian polishing. Following particle polishing, signal outside the masked actomyosin complex (myosin and the central three actin subunits) was subtracted. The subtracted particles then underwent 2 rounds of focused classification on the myosin motor domain leading to a final selection of 21,890 particles. The final 3D refinement was then performed using non-uniform refinement in cryoSPARC 3.2.0 (ref. 48).
Pseudo-atomic models
Homology pseudo-atomic models for primed and postPS actomyosin-5 structures were generated using Modeller in Chimera on the basis of the Protein Data Bank (PDB) files shown in Extended Data Table 1 (refs. 49,50). Each model comprised three actin subunits and one myosin-5 motor domain. Refinement of these models was performed using Coot51, with subsequent refinement of the nucleotide pocket in ISOLDE, implementing the hydrogen bonding coordination to the Pi groups as described in ref. 5 and Y119 coordination as described in ref. 18 as harmonic restraints during flexible fitting28. Real-space refinement was performed using Phenix52.
To permit elucidation of interactions occurring at the actomyosin interface, we used MD simulations as a further model refinement tool. These were performed with the Amber FF14SB forcefield and a GBSA (generalized Born with solvent-accessible surface area) implicit solvent model following the method described in ref. 22 with position restraints on all backbone atoms using a restraint weight of 10 kcal mol−1 Å−2 and a production run time of 3 ns. Interactions that were observed for at least half of the simulation time were included in the pseudo-atomic model. The D-helix and the actin N-terminal interactions with myosin were further refined by use of ISOLDE28 using default parameters and implementing the hydrogen bonding coordination to the nucleotide as described in refs. 5,18 as harmonic restraints during flexible fitting of the D-helix.
To enable interpretation of our mutant rigor actomyosin-5 cryo-EM density map, we rigidly fitted existing rigor actomyosin-5 PDB structures generated by ref. 18, into the map. The myosin chain from PDB ID 7PLV was well accommodated into the map, especially within the converter region (Extended Data Fig. 6f). Therefore, we used this as a model for our rigor myosin-5 structure to enable us to compare it to our postPS actomyosin-5 structure (Extended Data Fig. 6f,g).
To illustrate our proposed mechanism of force generation and actin activation (Supplementary Video 4), we separated out the motions of cleft closure and power stroke into a suggested time sequence. To achieve this, a chimeric model of primed and postPS actomyosin was generated to represent a midway point in the primed-to-postPS transition of myosin. This included myosin chain numbering: amino acids 1–128 primed, amino acids 129–449 postPS, amino acids 450–507 primed, amino acids 508–632 postPS, amino acids 633–763 primed. Structures were visualized in Chimera. Videos were generated by use of Chimera, Adobe Aftereffects and Adobe Premiere.
MD simulations
We originally used implicit solvent MD simulations, with short (3 ns) production run times, to establish interactions that may be occurring at the actomyosin interface. To test whether the side-chain interactions observed in our primed and postPS actomyosin models at the interface between actin and loop 2 and between actin and helix W persist over longer times when subjected to thermal fluctuations, we performed longer MD simulations (>100 ns; wall clock time of about 5 days), with three independent replicates in explicit solvent conditions for each model. All simulations were run in AMBER16 (refs. 53,54; GPU version PMEMD-CUDA) on all atom systems parameterized with the charmm36m forcefield55 and built using CHARMM-GUI56. The primed and postPS actomyosin structures were prepared for MD simulations using the CHARMM-GUI solution builder56,57,58. The ADP present in both structures and the Pi present in the primed structure were parameterized from the CHARMM-GUI library with the Pi built as H2PO4. All N termini present in the models were acetylated and C termini were made neutrally charged. A TIP3P octahedral water box (Supplementary Fig. 7) was built for each structure using 25 mM KCl, 38 mM potassium acetate and 2 mM MgCl2 (pH 7.0) with a diameter of 186 Å for primed and 172 Å for postPS actomyosin; each system had a total atom count of 468,219 and 369,572 for primed and postPS, respectively. The simulations were then run at a constant temperature of 300 K and constant pressure ensemble (NPT). Simulations were run with position restraints on all backbone atoms using a restraint weight of 5 kcal mol−1 Å−2, allowing side chains to move. Each condition was run in triplicate using a different random seed to initiate the starting velocities. Trajectories (Supplementary Videos 5 and 6) were analysed using Chimera, and distance plots for atom–atom interaction distances were prepared using GraphPad Prism (Extended Data Fig. 2). The percentage of time that interacting atoms were within H-bonding distance (3.3 Å) was outputted (Extended Data Table 2).
Myosin-5 full-length lever model
A model F-actin filament 17 subunits long was created by 7 superpositions of our 3-actin-subunit model. Full-length levers (to residue 909) were added onto our primed and postPS actomyosin structures by super-imposing levers from PDB ID 7YV9 chain A, aligned on the converter domain (residues 699–750). Lever swing and azimuthal displacement were measured using the measurement tools in Chimera.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.