Plant growth and high-molecular-weight DNA isolation
Twenty-five seeds each from the selected accessions (Supplementary Tables 1 and 7) were sown on 16-cm-diameter pots with compost soil. Plants were grown under greenhouse conditions with sodium halogen artificial 21â°C in the day for 16âh and 18â°C at night for 8âh. Leaves (8âg) were collected from 7-day-old seedlings, ground with liquid nitrogen to a fine powder and stored at â80â°C.
High-molecular-weight (HMW) DNA was purified from the powder, essentially as described56. In brief, nuclei were isolated, digested with proteinase K and lysed with SDS. Here, a standard watercolour brush with synthetic hair (size 8) was used to re-suspend the nuclei for digestion and lysis. HMW DNA was purified using phenolâchloroform extraction and precipitation with ethanol as described56. Subsequently, the HMW DNA was dissolved in 50âml of TE (pH 8.0) and precipitated by the addition of 5âml of 3âM sodium acetate (pH 5.2) and 100âml of ice-cold ethanol. The suspension was mixed by slow circular movements resulting in the formation of a white precipitate (HMW DNA), which was collected using a wide-bore 5âml pipette tip and transferred for 30âs into a tube containing 5âml of 75% ethanol. The washing was repeated twice. The HMW DNA was transferred into a 2âml tube using a wide-bore tip, collected with a polystyrene spatula, air-dried in a fresh 2âml tube and dissolved in 500âµl of 10âmM Tris-Cl (pH 8.0). For quantification, the Qubit dsDNA High Sensitivity Assay Kit (Thermo Fisher Scientific) was used. The DNA size-profile was recorded using the Femto Pulse system and the Genomic DNA 165âkb kit (Agilent). In typical experiments the peak of the size-profile of the HMW DNA for library preparation was around 165âkb.
DNA library preparation and PacBio HiFi sequencing
For fragmentation of the HMW DNA into 20âkb fragments, a Megaruptor 3 device (speed: 30) was used (Diagenode). A minimum of two HiFi SMRTbell libraries were prepared for each barley genotype following essentially the manufacturerâs instructions and the SMRTbell Express Template Prep Kit (Pacific Biosciences). The final HiFi libraries were size-selected (narrow-size range: 18â21âkb) using the SageELF system with a 0.75% Agarose Gel Cassette (Sage Sciences) according to standard manufacturer protocols.
HiFi circular consensus sequencing (CCS) reads were generated by operating the PacBio Sequel IIe instrument (Pacific Biosciences) following the manufacturerâs instructions. Per genotype, about four 8M SMRT cells (average yield: 24âgigabases HiFi CCS per 8M SMART cell) were sequenced to obtain an approximate haploid genome coverage of about 20-fold. In typical experiments the concentration of the HiFi library on plate was 80â95âpM. We used 30âh movie time, 2âh pre-extension and sequencing chemistry v.2.0. The resulting raw data were processed using the CCS4 algorithm (https://github.com/PacificBiosciences/ccs).
Hi-C library preparation and Illumina sequencing
In situ Hi-C libraries were prepared from 1-week-old barley seedlings on the basis of the previously published protocol13. Dovetail Omni-C data were generated for Bowman, Aizu6, Golden Melon and 10TJ18 as per the manufacturerâs instructions (https://dovetailgenomics.com/products/omni-c-product-page/). Sequencing and Hi-C raw data processing was performed as described before57,58.
Genome sequence assembly and validation
PacBio HiFi reads were assembled using hifiasm (v.0.11-r302)59. Pseudomolecule construction was done with the TRITEX pipeline60. Chimeric contigs and orientation errors were identified through manual inspection of Hi-C contact matrices. Genome completeness and consensus accuracy were evaluated using Merqury (v.1.3)61. Levels of duplication and heterozygosity were assessed with Merqury and FindGSE (v.1.94)62. Further, we estimated heterozygosity in the HiFi reads with a k-mer approach. We selected 35,202 bi-allelic SNPs from a genebank genomic study3. For each SNP we extracted the flanking sequences (±15âbp) from the SNP positions and put either SNP in the middle to obtain 31-mers for the reference and alternative alleles. The FASTA sequences of the k-mers are available from https://bitbucket.org/ipkdg/het_estimation. We counted the occurrence of these k-mers in the HiFi FASTQ files using BBDuk (https://jgi.doe.gov/data-and-tools/software-tools/bbtools/bb-tools-user-guide/bbduk-guide/) with the parameter ârpkmâ. Cenotype calling and the heterozygosity estimation were done in R. The full workflow is available from https://bitbucket.org/ipkdg/het_estimation.
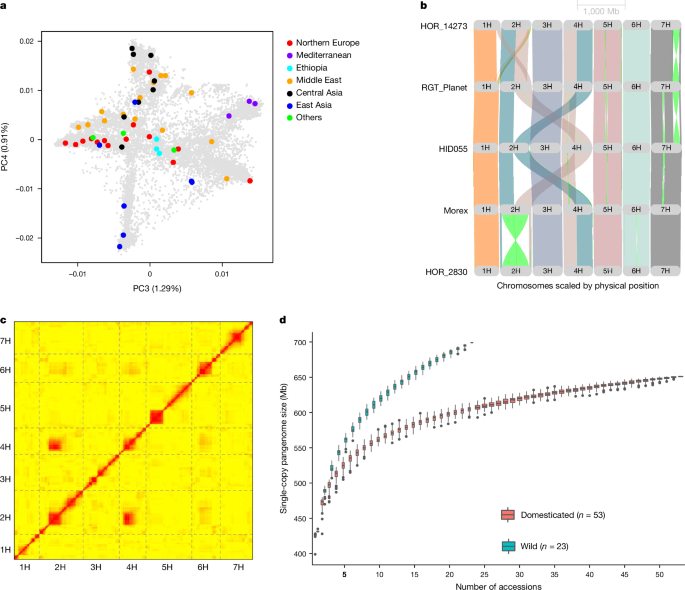
Single-copy pangenome construction
The single-copy regions in each chromosome-level assembly were identified by filtering 31-mers occurring more than once in the genomic regions by BBDuk (BBMap_37.93, https://jgi.doe.gov/data-and-tools/software-tools/bbtools). BBMap was used to count k-mer occurrences in each genome with the parameter âmincount 2. Then, non-unique genomic regions (that is, those composed of k-mers occurring at least twice) were masked by BBDuk on the basis of k-mer counts. Single-copy regions extracted in BED format and their sequences (with the command âbedtools complementâ) were retrieved using BEDTools (v.2.29.2)63. The single-copy sequences were clustered using MMseqs2 (Many-against-Many sequence searching)64 with the parameters â–cluster-modeâ and setting over 95% sequence identity. A representative from each cluster (the largest in a cluster) was selected to estimate the pangenome size.
Illumina resequencing
A total of 1,000 PGRs and 315 elite barley cultivars (Supplementary Table 6) were used for whole-genome resequencing. Illumina Nextera libraries were prepared and sequenced on an Illumina NovaSeq 6000 at IPK Gatersleben (Supplementary Table 6).
SNP and SV calling
Reciprocal genome alignment, in which each of the pangenome assemblies was aligned to the MorexV3 assembly with the latter acting either as alignment query or reference, was done with Minimap2 (v.2.20)65. From the resultant two alignment tables, indels were called by Assemblytics (v.1.2.1)66 and only deletions were selected in both alignments to convert into presence/absence variants relative to the Morex reference genome. Further, balanced rearrangements (inversions, translocations) were scanned for with SyRI67. To call SNPs, raw sequencing reads were trimmed using cutadapt (v.3.3)68 and aligned to the MorexV3 reference genome using Minimap2 (v.2.20)65. The resulting alignments were sorted with Novosort (v.3.09.01) (http://www.novocraft.com). BCFtools (v.1.9)69 was used to call SNPs and short indels. A genome-wide association study was performed in GEMMA (v.0.98.1)70 using default parameters with a mixed linear model and an estimated kinship matrix. Read depth was calculated at each complex locus in each accession. The raw HiFi reads were aligned to the respective genome using minimap2 (ref.â71) and the median depth per locus was calculated using mosdepth (v.0.2.6)72.
Linkage disequilibrium in the Barke x HID055 population
Linkage disequilibrium between each pair of SNPs (both intrachromosomal and interchromosomal) was calculated as the squared Pearson product-moment correlation between the quantitative identity-by-descent (IBD) matrix scores presented in Additional File 1 of ref.â73 (https://datadryad.org/stash/dataset/doi:10.5061/dryad.36rm1). The linkage disequilibrium plot was created with SAS PROC TEMPLATE and SGRENDER (SAS Institute) on the genetic map from ref.â18.
Preparation and Illumina sequencing of narrow-size whole-genome sequencing libraries for core50
First, 10âµg of DNA in 130âµl was sheared in tubes (Covaris microTUBE AFA Fiber Pre-Slit Snap Cap) to an average size of approximately 250âbp using a Covaris S220 focused-ultrasonicator (peak incidence power: 175âW, duty factor: 10%; cycles per burst: 200; time: 180âs) according to standard manufacturer protocols (Covaris). The sheared DNA was size-selected using a BluePippin device and a 1.5% agarose cassette with internal R2 marker (Sage Sciences). A tight size setting at 260âbp was used for the purification of fragments in the narrow range of 200â300âbp (typical yield: 1â3âµg). The size-selected DNA was used for the preparation of PCR-free whole-genome sequencing (WGS) libraries using the Roche KAPA Hyper Prep kit according to the manufacturerâs protocols (Roche Diagnostics). A total of 10â12 libraries were provided with unique barcodes, pooled at equimolar concentrations and quantified by quantitative PCR using the KAPA Library Quantification Kit for Illumina Platforms according to standard protocols (Roche Diagnostics). The pools were sequenced (2âÃâ151âbp, paired-end) using four S4 XP flowcells and the Illumina NovaSeq 6000 system (Illumina) at IPK Gatersleben.
Contig assembly of core50 sequencing data
Raw reads were demultiplexed on the basis of index sequences and duplicate reads were removed from the sequencing data using Fastuniq74. The read1 and read2 sequences were merged on the basis of the overlap using bbmerge.sh from bbmap (v.37.28)75. The merged reads were error-corrected using BFC (v.181)76. The error-corrected merged reads were used as an input for Minia3 (v.3.2.0)77 to assemble reads into unitigs with the following parameters, -no-bulge-removal -no-tip-removal -no-ec-removal -out-compress 9 -debloom original. The Minia3 source was assembled to enable k-mer size up to 512 as described in the Minia3 manual. Iterative Minia3 runs with increasing k-mer sizes (100, 150, 200, 250 and 300) were used for assembly generation as provided in the GATB Minia pipeline (https://github.com/GATB/gatb-minia-pipeline). In the first iteration, k-mer size of 50 was used to assemble input reads into unitigs. In the next runs, the input reads as well as the assembly of the previous iteration were used as input for the Minia3 assembler. BUSCO analysis was conducted on the contig assemblies using BUSCO (v.3.0.2) with embryophyta_odb9 dataset14. In addition, high-confidence gene models from the Morex V3 reference9 were aligned to the contig assemblies to assess completeness, with the parameters of greater than or equal to 90% query coverage and greater than or equal to 97% identity.
Pangenome accessions in diversity space
Pseudo-FASTQ paired-end reads (tenfold coverage) were generated from the 76 pangenome assemblies with fastq_generator (https://github.com/johanzi/fastq_generator) and aligned to the MorexV3 reference genome sequence assembly9 using Minimap2 (v.2.24-r1122, ref.â65). SNPs were called together with short-read data (Supplementary Table 6) using BCFtools78 v.1.9 with the command âmpileup -q 20 -Q20 –excl-flags 3332â. To plot the diversity space of cultivated barley, the resultant variant matrix was merged with that of 19,778 domesticated barleys from ref.â3 (genotyping-by-sequencing (GBS) data). SNPs with more than 20% missing or more than 20% heterozygous calls were discarded. Principal component analysis was done with smartpca79 v.7.2.1. To represent the diversity of wild barleys, we used published GBS and WGS data of 412 accessions of that taxon8,54. Variant calling for GBS data was done with BCFtools78 (v.1.9) using the command âmpileup -q 20 -Q20â. The resultant variant matrix was filtered as follows: (1) only bi-allelic SNP sites were kept; (2) homozygous genotype calls were retained if their read depth was greater than or equal to 2 and less than or equal to 50 and set to missing otherwise; (3) heterozygous genotype calls were retained if the read depth of both alleles was greater than or equal to 2 and set to missing otherwise. SNPs with more than 20% missing, more than 20% heterozygous calls or a minor allele frequency below 5% were discarded. Principal component analysis was done with smartpca79 v.7.2.1. A matrix of pairwise genetic distances on the basis of identity-by-state (IBS) was computed with Plink2 (v.2.00a3.3LM, ref.â80) and used to construct a neighbour-joining tree with Fneighbor (http://emboss.toulouse.inra.fr/cgi-bin/emboss/fneighbor) in the EMBOSS package81. The tree was visualized with Interactive Tree Of Life (iTOL)82.
Haplotype representation
Pangenome assemblies were mapped to MorexV3 as described above (âPangenome accessions in diversity spaceâ). Read depth was calculated with SAMtools78 v.1.16.1. Genotype calls were set to missing if they were supported by fewer than two reads. IBS was calculated with PIink2 (v.2.000a3.3LM, ref.â80) in 1âMb windows (shift: 0.5âMb) using the using command â–sample-diff counts-only counts-cols=ibs0, ibs1â. Windows that in one of both accessions in the comparison had twofold coverage over less than 200âkb were set to missing. The number of differences (d) in a window was calculated as ibs0â+âibs1/2, where ibs0 is the number of homozygous differences and ibs1 that of heterozygous ones. This distance was normalized for coverage by the formula d/iâÃâ1âMb, where i is the size in bp of the region covered in both accessions in the comparison that had at least twofold coverage. In each window, we determined for each among the PGRs and cultivars panel the closest pangenome accession according to the coverage-normalized IBS distance. Only accessions with fewer than 10% missing windows due to low coverage were considered, leaving 899 PGRs and 264 cultivars.
The distance to the closest pangenome accession was plotted with the R package ggplot2 to determine the threshold for similarity (Extended Data Fig. 2d).
Transcriptome sequencing for gene annotation
Data for transcript evidence-based genome annotation were provided by the International Barley Pan-Transcriptome Consortium, and a detailed description of sample preparation and sequencing is provided elsewhere83. In brief, the 20 genotypes sequenced for the first version of the barley pangenome8 were used for transcriptome sequencing. Five separate tissues were sampled for each genotype. These were: embryo (including mesocotyl and seminal roots), seedling shoot, seedling root, inflorescence and caryopsis. Three biological replicates were sampled from each tissue type, amounting to 330 samples. Four samples failed quality control and were excluded.
Preparation of the strand-specific dUTP RNA-seq libraries and Illumina paired-end 150âbp sequencing were carried out by Novogene. In addition, PacBio Iso-Seq sequencing was carried out using a PacBio Sequel IIe sequencer at IPK Gatersleben. For this, a single sample per genotype was obtained by pooling equal amounts of RNA from a single replicate from all five tissues. Each sample was sequenced on an individual 8M SMRT cell.
De novo gene annotation
Structural gene annotation was done by combining de novo gene calling and homology-based approaches with RNA-seq, Iso-Seq and protein datasets (Extended Data Fig. 3a). Using evidence derived from expression data, RNA-seq data were first mapped using STAR84 (v.2.7.8a) and subsequently assembled into transcripts by StringTie85 (v.2.1.5, parameters -m 150-t -f 0.3). Triticeae protein sequences from available public datasets (UniProt86, https://www.uniprot.org, 10 May 2016) were aligned against the genome sequence using GenomeThreader87 (v.1.7.1; arguments -startcodon -finalstopcodon -species rice -gcmincoverage 70 -prseedlength 7 -prhdist 4). Iso-Seq datasets were aligned to the genome assembly using GMAP88 (v.2018-07-04). All assembled transcripts from RNA-seq, Iso-Seq and aligned protein sequences were combined using Cuffcompare89 (v.2.2.1) and subsequently merged with StringTie (v.2.1.5, parameters –merge -m150) into a pool of candidate transcripts. TransDecoder (v.5.5.0; http://transdecoder.github.io) was used to identify potential ORFs and to predict protein sequences within the candidate transcript set.
Ab initio annotation was initially done using Augustus90 (v.3.3.3). GeneMark91 (v.4.35) was additionally used to further improve structural gene annotation. To avoid potential over-prediction, we generated guiding hints using the above-described RNA-seq, protein and Iso-Seq datasets as described before92. A specific Augustus model for barley was built by generating a set of gene models with full support from RNA-seq and Iso-Seq. Augustus was trained and optimized following a published protocol92. All structural gene annotations were joined using EVidenceModeller93 (v.1.1.1), and weights were adjusted according to the input source: ab initio (Augustus: 5, GeneMark: 2), homology-based (10). Additionally, two rounds of PASA94 (v.2.4.1) were run to identify untranslated regions and isoforms using the above-described Iso-Seq datasets.
We used BLASTP95 (ncbi-blast-2.3.0+, parameters -max_target_seqs 1 -evalue 1eâ05) to compare potential protein sequences with a trusted set of reference proteins (Uniprot Magnoliophyta, reviewed/Swissprot, downloaded on 3 August 2016; https://www.uniprot.org). This differentiated candidates into complete and valid genes, non-coding transcripts, pseudogenes and TEs. In addition, we used PTREP (release 19; http://botserv2.uzh.ch/kelldata/trep-db/index.html), a database of hypothetical proteins containing deduced amino acid sequences in which internal frameshifts have been removed in many cases. This step is particularly useful for the identification of divergent TEs with no significant similarity at the DNA level. Best hits were selected for each predicted protein from each of the three databases. Only hits with an e-value below 10âÃâ10â10 were considered. Furthermore, functional annotation of all predicted protein sequences was done using the AHRD pipeline (https://github.com/groupschoof/AHRD).
Proteins were further classified into two confidence classes: high and low. Hits with subject coverage (for protein references) or query coverage (transposon database) above 80% were considered significant and protein sequences were classified as high-confidence using the following criteria: protein sequence was complete and had a subject and query coverage above the threshold in the UniMag database or no BLAST hit in UniMag but in UniPoa and not PTREP; a low-confidence protein sequence was incomplete and had a hit in the UniMag or UniPoa database but not in PTREP. Alternatively, it had no hit in UniMag, UniPoa or PTREP, but the protein sequence was complete. In a second refinement step, low-confidence proteins with an AHRD score of 3* were promoted to high-confidence.
Gene projections
Gene contents of the remaining 56 barley genotypes were modelled by the projection of high-confidence genes on the basis of evidence-based gene annotations of the 20 barley genotypes described above. The approach was similar to and built upon a previously described method8. To reduce computational load, 760,078 high-confidence genes of the 20 barley annotations were clustered by cd-hit96 requiring 100% protein sequence similarity and a maximal size difference of four amino acids. The resulting 223,182 source genes were subsequently used for all downstream projections as the non-redundant transcript set representative for the evidence-based annotations. For each source, its maximal attainable score was determined by global protein self-alignment using the NeedlemanâWunsch algorithm as implemented in Biopython97 v.1.8 and the blosum62 substitution matrix98 with a gap open and extension penalty of 0.5 and 10.0, respectively.
Next, we surveyed each barley genome sequence using minimap2 (ref.â65) with options â-ax splice:hqâ and â-ufâ for genomic matches of source transcripts. Each match was scored by its pairwise protein alignment with the source sequence that triggered the match. Only complete matches with start and stop codons and a score greater than or equal to 0.85 of the source self-score (see above) were retained. The source models were classified into four bins by decreasing confidence qualities: with or without pfam domains, plastid- and transposon-related genes. Projections were performed stepwise for the four qualities, starting from the highest to the lowest. In each quality group, matches were then added into the projected annotation if they did not overlap with any previously inserted model by their coding region. Insertion order progressed from the top to the lowest scoring match. In addition, we tracked the number of insertions for each source by its identifier. For the two top quality categories, we performed two rounds of projections, first inserting each source maximally only once followed by rounds allowing one source inserted multiple times into the projected annotation. To consolidate the 20 evidence-based, initial annotations for any genes potentially missed, we used an identical approach but inserted any non-overlapping matches starting from the previous RNA-seq-based annotation. A detailed description of the projection workflow, parameters and code is provided at the GitHub repository (https://github.com/GeorgHaberer/gene_projection/tree/main/panhordeum). An overview of the projection scheme can be found in the parent directory of the repository. Because complex loci contain numerous pseudogenes, the loci were searched by BLASTN99 for sequences homologous to annotated genes but not present in the set of annotated genes. Pseudogenes were accepted if they covered at least 80% of a gene homologue.
Definition of core, cloud and shell genes
Phylogenetic HOGs on the basis of the primary protein sequences from 76 annotated barley genotypes were calculated using Orthofinder100 v.2.5.5 (standard parameters). The scripts for calculation of core/shell and cloud genes have been deposited in the repository https://github.com/PGSB-HMGU/BPGv2. Core HOGs contain at least one gene model from all 76 barley genotypes included in the comparison. Shell HOGs contain gene models from at least two barley genotypes and at most 75 barley genotypes. Genes not included in any HOG (âsingletonsâ), or clustered with genes only from the same genotype, were defined as cloud genes. GENESPACE101 was used to determine syntenic relationships between the chromosomes of all 76 genotypes.
Annotation of TEs
The 20 barley accessions with expression data were softmasked for transposons before the de novo gene detection using the REdat_9.7_Triticeae section of the PGSB transposon library102. Vmatch (http://www.vmatch.de) was used as matching tool with the following parameters: identityâ>â=70%, minimal hit length 75âbp, seedlength 12âbp (vmmatch -d -p -l 75 -identity 70 -seedlength 12 -exdrop 5 -qmaskmatch tolower). The percentage masked was around 84% and almost identical for all 20 accessions.
Full-length long terminal repeat retrotransposon candidate elements were detected de novo for each of the 76 barley accessions by their structural hallmarks with LTRharvest103 followed by LTRdigest104. Both programs are contained in genometools87 (http://github.com/genometools/genometools, v.1.5.10). LTRharvest identifies within the specified parameters long terminal repeats and target site duplications whereas LTRdigest was used to determine polypurine tracts and primer binding sites. The transfer RNA library needed as input for the primer binding sites was beforehand created by running tRNAscan-SE-1.3 (ref.â105) on each assembly. The parameter settings for LTRharvest were: â-overlaps best -seed 30 -minlenltr 100 -maxlenltr 2000 -mindistltr 3000 -maxdistltr 25000 -similar 85 -mintsd 4 -maxtsd 20 -motif tgca -motifmis 1 -vic 60 -xdrop 5 -mat 2 -mis -2 -ins -3 -del -3 -longoutputâ; for LTRdigest: â-pptlen 8 30 -uboxlen 3 30 -pptradius 30 -pbsalilen 10 30 -pbsoffset 0 10 -pbstrnaoffset 0 30 -pbsmaxedist 1 -pbsradius 30â. The insertion age of each long terminal repeat retrotransposon instance was calculated from the divergence of its 5â² and 3â² long terminal repeat sequences using a random mutation rate of 1.3âÃâ10â8 (ref.â106).
Whole-genome pangenome graphs
Genome graphs were constructed using Minigraph19 v.0.20-r559. Other graph construction tools (PGGB107, Minigraph-Cactus108) turned out to be computationally prohibitive for a genome of this size and complexity, combined with the large number of accessions used in this investigation. Minigraph does not support small variants (less than 50âbp), thus graph complexity is lower than with other tools. However, even with Minigraph, graph construction at the whole-genome level was computationally prohibitive and thus graphs had to be computed separately for each chromosome, precluding detection of interchromosomal translocations.
Graph construction was initiated using the Morex V3 assembly9 as a reference. The remaining assemblies were added into the graph sequentially, in order of descending dissimilarity to Morex. SVs were called after each iteration using gfatools bubble (v.0.5-r250-dirty, https://github.com/lh3/gfatools). Following graph construction, the input sequences of all accessions were mapped back to the graph using Minigraph with the â–callâ option enabled, which generates a path through the graph for each accession. The resulting BED format files were merged using Minigraphâs mgutils.js utility script to convert them to P lines and then combined with the primary output of Minigraph in the proprietary RGFA format (https://github.com/lh3/gfatools/blob/master/doc/rGFA.md). Graphs were then converted from RGFA format to GFA format (https://github.com/GFA-spec/GFA-spec/blob/master/GFA1.md) using the âconvertâ command from the vg toolkit109 v.1.46.0 âAltamuraâ. This step ensures that graphs are compatible with the wider universe of graph processing tools, most of which require GFA format as input. Chromosome-level graphs were then joined into a whole-genome graph using vg combine. The combined graph was indexed using vg index and vg gbwt, two components of the vg toolkit109.
General statistics for the whole-genome graph were computed with vg stats. Graph growth was computed using the heaps command from the ODGI toolkit110 v.0.8.2-0-g8715c55, followed by plotting with its companion script heaps_fit.R. The latter also computes values for gamma, the slope coefficient of Heapâs law which allows the classification of pangenome graphs into open or closed pangenomes, that is, a prediction of whether the addition of further accessions would increase the size of the pangenome111.
SV statistics were computed on the basis of the final BED file produced after the addition of the last line to the graph. A custom shell script was used to classify variants according to the Minigraph custom output format. This allows the extraction of simple, that is, non-nested, indels (relative to the MorexV3 graph backbone), as well simple inversions. The remaining SVs fall into the âcomplexâ category in which there can be multiple levels of nesting of different variant types and this precluded further, more fine-grained classification. To compute overlap with the SVs from Assemblytics, a custom script was used to extract the variant coordinates from both sets, and bedtools intersect63 was then used to compute their intersection on the basis of a spatial overlap of 70%.
To elucidate the effect of a graph-based reference on short-read mapping, we obtained WGS Illumina reads from five barley samples (Extended Data Fig. 4b) in the European Nucleotide Archive and mapped these onto the whole-genome graph using vg giraffe112. For comparison with the standard approach of mapping reads to a linear single genome reference, we mapped the same reads to the MorexV3 reference genome sequence assembly9 with bwa mem113 v.0.7.17-r1188. Mapping statistics were computed with vg109 stats and samtools78 stats (v.1.9), respectively.
To elucidate tool bias as a confounding factor in the comparison between the mappings, we first produced a linearized version of the pangenome graph using gfatools gfa2fa (https://github.com/lh3/gfatools) and then mapped the WGS reads from all five accessions to this new reference sequence, using BWA mem as before for the cv. Morex V3 reference sequence. This allows a more appropriate comparison between the single cultivar reference sequence and the pangenome sequence without being affected by algorithmic differences between the tools used (BWA/giraffe). Mappings were filtered to retain only reads with zero mismatches, using sambamba114. For the graph mappings, the âTotal perfectâ statistic from the vg stats output of the GAM files was used.
To investigate the srh1 paths in the pangenome graph, we first extracted all nodes from the graph into a FASTA file and then used the enhancer region identified in cv. Barke as associated with the long-haired srh1 phenotype (chr5H:496,182,748-496,187,020) as query in a BLAST search against the nodes. This recovered five nodes with an identity percentage value of greater than 98%. We then used vg find from the vg toolkit v.1.56.0 (ref.â109) to extract a subgraph from the full graph (with a graph context of five steps either side) using the node identifiers. The subgraph was then plotted using odgi viz from the ODGI toolkit v.0.8.3-26-gbc7742ed (ref.â110).
To genotype samples from the core800 collection against the srh1 region of the graph, we first identified a small set of four samples each with either the short- or long-haired phenotype, picked at random from a group of core800 samples that all shared the same WGS read depth (5Ã). These samples were HOR_1102, HOR_17654, HOR_4065, HOR_1264, HOR_14704, HOR_7629, HOR_17678 and HOR_11406. We then mapped their Illumina WGS reads to the full pangenome graph using vg giraffe112 and extracted a subgraph of the mappings with vg chunk109. The subgraph was then genotyped using vg pack and vg call with cv. Barke as the reference accession, following the approach proposed in ref.â115. Variants in the resulting VCF files were identified using a simple grep command with the identifiers of the five nodes recovered with the Barke sequence as described above. Scripts used here are available at https://github.com/mb47/minigraph-barley/tree/main/scripts/srh1_analysis.
Analysis of the Mla locus
The coordinates and sequences of the 32 genes present at the Mla locus were extracted from the MorexV3 genome sequence assembly9. To find the corresponding position and copy number in each of the 76 genomes, we used BLAST95 (-perc_identity: 90, -word_size: 11, all other parameters set as default). The expected BLAST result for a perfectly conserved allele is a long fragment (exon_1) of 2,015âbp followed by a gap of approximately 1,000âbp due to the intron and another fragment (exon_2) of 820âbp. To detect the number of copies, first multiple BLAST results for a single gene were merged if two different BLAST segments were within 1.1âkb. Then only if the total length of the input was found, this was counted as a copy. To analyse the structural variation across all 76 accessions, the non-filtered BLAST results were plotted in a region of â20,000 and +500,000âbase pairs around the start of the BPM gene HORVU.MOREX.r3.1HG0004540 that was used as an anchor (present in all 76 lines; Supplementary Figs. 5 and 6). To detect the different Mla alleles, three different thresholds of -Perc_identity for the BLAST were used: 100, 99 and 98.
Scan for structurally complex loci
We used a pipeline developed in ref.â27 that performs sequence-agnostic identification of long-duplication-prone regions (henceforth, complex regions) in a reference genome, followed by identification of gene families with a statistical tendency to occur within complex regions. The pipeline assumes that a candidate long, duplication-prone region will contain an elevated concentration of locally repeated sequences in the kb-scale length range. We first aligned the MorexV3 genome sequence assembly9 against itself using lastz116 (v.1.04.03; arguments: â–notransition –step=500 –gappedâ). For practicality purposes, this was done in 2âMb blocks with a 200âkb overlap, and any overlapping complex regions identified in multiple windows were merged. For each window, we ignored the trivial end-to-end alignment, and, of the remaining alignments, retained only those longer than 5âkb and falling fully within 200âkb of one and another. An alignment âdensityâ was calculated over the chromosome by calculating, at âinterrogation pointsâ spaced equally at 1âkb intervals along the length of the chromosome, an alignment density score which is simply the sum of all the lengths of any of the filtered alignments spanning that interrogation point. A Gaussian kernel density (bandwidth 10âkb) was calculated over these interrogation points, weighted by their scores. To allow comparability between windows, the interrogation point densities were normalized by the sum of scores in the window. Runs of interrogation points at which the density surpassed a minimum density threshold were flagged as complex regions. A few minor adjustments to these regions (merging of overlapping regions, and trimming the end coordinates to ensure the stretches always begin and end in repeated sequence) yielded the final tabulated list of complex regions and their positions in the MorexV3 genome assembly (Supplementary Table 8). The method was implemented in R, making use of the package data.table. Genes in each long, duplication-prone region were clustered with UCLUST117 (v.11, default parameters) using a protein clustering distance cutoff of 0.5 and for each cluster the most frequent functional description as per the MorexV3 gene annotation9 was assigned as the functional description of the cluster. Self-alignment for characterization of evolutionary variability (Supplementary Fig. 7) was performed using lastz116 (v.1.04.03; settings â–self –notransition –gapped –nochain –gfextend –step=50â).
Molecular dating of divergence times of duplicated genes in complex loci
For molecular dating of gene duplications, we used segments of up to 4âkb, starting 1âkb upstream of duplicated genes in complex loci. With this, we presumed only to use intergenic sequences which are free from selection pressure and thus evolve at a neutral rate of 1.3âÃâ10â8 substitutions per site per year106. The upstream sequences of all duplicated genes of the respective complex locus were then aligned pairwise with the program Water from the EMBOSS package81 (obtained from Ubuntu repositories, https://ubuntu.com). This was done for all gene copies of all barley accession for which multiple gene copies were found. Molecular dating of the pairwise alignments was done as previously described118 using the substitution rate of 1.3âÃâ10â8 substitutions per site per year106.
Amy1_1 analysis in pangenome assemblies
The amy1_1 gene copy HORVU.MOREX.PROJ.6HG00545380 was used for BLAST against all 76 genome assemblies. Full-length sequences with identity over 95% were extracted and used for further analyses. Unique sequences were identified by clustering at 100% identity using CD-Hit96 and were aligned using MAFFT119 v.7.490. Sequence variants among amy1_1 gene copies at genomic DNA, coding sequence (CDS) and respective protein level were collected and amy1_1 haplotypes (that is, the combinations of copies) in each genotype assembly were summarized using R120 v.4.2.2. A Barke-specific SNP locus (GGCGCCAGGCATGATCGGGTGGTGGCCAGCCAAGGCGGTGACCTTCGTGGACAACCACGACACCGGCTCCACGCAGCACATGTGGCCCTTCCCTTCTGACA[A/G]GGTCATGCAGGGATATGCGTACATACTCACGCACCCAGGGACGCCATGCATCGTGAGTTCGTCGTACCAATACATCACATCTCAATTTTCTTTTCTTGTTTCGTTCATAA) for amy1_1 haplotype cluster ProtHap3 (Supplementary Table 21) was identified and used for KASP marker development (LGC Biosearch Technologies).
Comparative analysis of the amy1_1 locus structure
On the basis of the genome annotation of cv. Morex, 15 gene sequences on either side of amy1_1 gene copy HORVU.MOREX.PROJ.6HG00545440 were extracted. The 31 genes were compared against the 76 genome assemblies using NCBI-BLAST95 (BLASTN, word_size of 11 and percent identity of 90, other parameters as default). Alignment plots were generated from the BLAST result coordinates by scaling on the basis of the mid-point between HORVU.MOREX.r3.6HG0617300/HORVU.MOREX.PROJ.6HG00545250 and HORVU.MOREX.r3.6HG0617710/HORVU.MOREX.PROJ.6HG00545670. All BLAST results in the region (±1âMb) around this mid-point were plotted using R120.
Amy1_1 PacBio amplicon sequencing
Genomic DNA from 1-week-old Morex seedling leaves was extracted with DNeasy Plant Mini Kit (QIAGEN). On the basis of the MorexV3 genome sequence assembly9, amy1_1 full-length copy-specific primers were designed using Primer3 (ref.â121) (https://primer3.ut.ee/): 6F: GTAGCAGTGCAGCGTGAAGTC; 80F: AGACATCGTTAACCACACATGC; 82F: GTTTCTCGTCCCTTTGCCTTAA; 82F: GTTTCTCGTCCCTTTGCCTTAA; 33R: GATCTGGATCGAAGGAGGGC; 79R: TCATACATGGGACCAGATCGAG; 80R: ACGTCAAGTTAGTAGGTAGCCC. All forward primers were tagged with bridge sequence (preceding T to primer name) [AmC6]gcagtcgaacatgtagctgactcaggtcac, whereas reverse primers were tagged with [AmC6]tggatcacttgtgcaagcatcacatcgtag to allow annealing to barcoding primers. These bridge sequence-tagged gene-specific primers were used in pairs with each other, targeting 1â2 copies of 3â6âkb amy1_1 genes, including upstream and downstream 500â1000âbp regions: T6Fâ+âT33R, T6Fâ+âT79R, T80Fâ+âT80R and T82Fâ+âT80R. A two-step PCR protocol was conducted. The first step PCR reaction was prepared in a 25âμl volume using 2âμl of DMSO, 0.3âμl of Q5 polymerase (New England Biolabs), 1âμl of amy1_1-specific primer pair (10âμM each), 2âμl of gDNA, 0.5âμl of dNTPs (10âmM), 5âμl of Q5 buffer and H2O. The PCR programme was as follows: initial denaturation at 98â°C/1âmin followed by 25â28 cycles of 98â°C/30âs, 58â°C/30âs and 72â°C/3âmin for extension, with a final extension step of 72â°C/2âmin. The second PCR step (barcoding PCR) was prepared in the same way using 1âμl of the first PCR product as DNA template, barcoding primers (Pacific Biosciences) and the PCR programme reduced to 20 cycles. After quality check on 1% agarose gel, all barcoded PCR products were mixed and purified with AMPure PB (Pacific Biosciences). The SMRT bell library preparation and sequencing were carried out at BGI Tech Solutions. Sequencing data were analysed using SMRT Link v.10.2. To minimize PCR chimeric noise, CCSs were first constructed for each molecule. Second, long amplicon analysis was carried out on the basis of subreads from 50âbp windows spanning peak positions of all CCS length. Final consensus sequences for each amy1_1 were determined with the aid of size estimation from agarose gel imaging.
Amy1_1 SNP haplotype analysis and k-mer-based copy number estimation
SNP haplotypes were analysed in 1,315 PGRs and elite cultivars in the extended amy1_1 cluster region (MorexV3 chr6H: 516,385,490â517,116,415âbp). SNPs with more than 20% missing data among the analysed lines and minor allele frequency less than 0.01 were removed from downstream analyses. The data were converted to 0, 1 and 2 format using VCFtools122 and samples were clustered using the pheatmap package (https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf) from R statistical environment57. The sequential clustering approach was used to achieve the desired separation. At each step, two extreme clusters were selected and then samples from each cluster were clustered separately. The process was repeated until the desired separation was achieved on the basis of visual inspection.
K-mers (kâ=â21) were generated from the Morex amy1_1 gene family membersâ conserved region using jellyfish123 v.2.2.10. After removing k-mers with counts from regions other than amy1_1 in the Morex V3 genome assembly, k-mers were counted in the Illumina raw reads (Supplementary Table 6) using Seal (BBtools, https://jgi.doe.gov/data-and-tools/software-tools/bbtools/). All k-mer counts were normalized to counts per MorexV3 genome and amy1_1 copy number was estimated as the median count of all k-mers from each accession in R.
Estimation ability was validated by comparing copy number from pangenome assemblies and short-read sequencing data (Extended Data Fig. 8c). For 1,000 PGRs, countries (with at least 10 accessions) were colour-shaded on the basis of their proportions of accessions with amy1_1 copy number greater than 5 on a world map using the R package maptools (https://cran.r-project.org/web/packages/maptools/index.html).
To construct a network from SNP haplotypes, all 371 amy1_1 copies (except ORF 89, 90 and 93; Supplementary Table 14) were aligned using MAFFT119 v.7.490. Median-joining haplotype networks were generated using PopART124 with an epsilon value of 0.
Local pangenome graph for amy1_1
The coordinates of amy1_1 copies in 76 genome assemblies were obtained by BLAST searches with the Morex allele of HORVU.MOREX.PROJ.6HG00545380. The genomic intervals surrounding amy1_1 from 10âkb upstream of the first copy to 10âkb downstream of the last copy were extracted from corresponding assemblies and used for further analyses. We applied PGGB (v.0.4.0, https://github.com/pangenome/pggb) for 76 amy1_1 sequences with parameters â-n 76 -t 20 -p 90 -s 1000 -Nâ. The graph was visualized using Bandage125 (v.0.8.1). ODGI (v.0.7.3, command âpathsâ)110 was used to get a sparse distance matrix for paths with the parameter â-dâ. The resultant distance matrix was plotted with the R package pheatmap (https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf). Six representative sequences of amy1_1 were aligned against Morex by BLAST+ (v.2.13.0)99.
AMY1_1 protein structure and protein folding simulation
The published protein structure of α-amylase AMY1_1 from accession Menuet, in complex with the pseudo-tetrasaccharide acarbose (PDB: 1BG9; ref.â42), was used to simulate the structural context of the amino acid variants identified in barley accessions Morex, Barke and RGT Planet. The amino acid sequences of the crystalized AMY1_1 protein from Menuet and the Morex reference copy amy1_1 HORVU.MOREX.PROJ.6HG00545380 used in this study are identical. The protein was visualized using PyMol 2.5.5 (Schrödinger). The Dynamut2 webserver126 was used to predict changes in protein stability and dynamics by introducing amino acid variants identified in the Morex, Barke and RGT Planet genome assemblies.
Development of diverse amy1_1 haplotype barley NILs
NILs with different amy1_1 haplotypes were derived from crosses between RGT Planet as recipient and Barke or Morex amy1_1 cluster donor parents (ProtHap3, ProtHap4 and ProtHap0, respectively; Supplementary Table 21), followed by two subsequent backcrosses to RGT Planet and one selfing step (BC2S1) to retrieve homozygous plants at the amy1_1 locus. A total of four amy_1_1âBarke NILs (ProtHap3) and one amy1_1âMorex NIL (ProtHap0) were developed and tested against RGT Planet (ProtHap4) replicates. Plants were grown in a greenhouse at 18â°C under 16/8-h light/dark cycles. Foreground and background molecular markers were used in each generation to assist plant selection. Respective BC2S1 plants were genotyped with the Barley Illumina 15K array (SGS Institut Fresenius, TraitGenetics Section, Germany) and grown to maturity. Grains were collected and further propagated in field plots in consecutive years in various locations (Nørre Aaby, Denmark; Lincoln, New Zealand; Maule, France). Grains from field plots were collected and threshed using a Wintersteiger Elite plot combiner, and sorted by size (threshold, 2.5âmm) using a Pfeuffer SLN3 sample cleaner (Pfeuffer).
Micro-malting and α-amylase activity analysis
Non-dormant barley samples of RGT Planet and respective NILs with different amy1_1 haplotypes (50âg each, graded greater than 2.5âmm) were micro-malted in perforated stainless-steel boxes. The barley samples were steeped at 15â°C by submersion of the boxes in water. Steeping took place for 6âh on day one, 3âh on day two and 1âh on day three, followed by air rests, to reach 35%, 40% and 45% water content, respectively. The actual water uptake of individual samples was determined as the weight difference between initial water content, measured with a Foss 1241 NIT instrument, and the sample weight after surface water removal. During air rest, metal beakers were placed into a germination box at 15â°C. Following the last steep, the barley samples were germinated for 3âd at 15â°C. Finally, barley samples were kiln-dried in an MMK Curio kiln (Curio Group) using a two-step ramping profile. The first ramping step started at a set point of 27â°C with a linear ramping at 2â°Câhâ1 to the breakpoint at 55â°C using 100% fresh air. The second linear ramping was at 4â°Câhâ1, reaching a maximum at 85â°C. This temperature was kept constant for 90âmin using 50% air recirculation. The kilned samples were then deculmed using a manual root removal system (Wissenschaftliche Station für Brauerei). α-Amylase activity was measured using the Ceralpha method (Ceralpha Method MR-CAAR4, Megazyme) modified for Gallery Plus Beermaster (Thermo Fisher Scientific).
Amy1_1 gene expression of RGT Planet and amy1_1âBarke NIL during micro-malting
Samples (50âg each, graded greater than 2.5âmm) were micro-malted as described in the previous section. During micro-malting, grains were sampled at 24âh, 48âh and 72âh. Grain samples were first freeze-dried at â80â°C and then milled at room temperature. Total RNA was isolated from 20â200âmg of flour using the Spectrum Plant Total RNA Kit (Sigma Aldrich) and cleaned using RNA Clean & Concentrator (ZYMO Research) following a published protocol127. For RNA-seq analysis, libraries were prepared and single-end sequenced with a length of 75âbp as described in ref.â127. Gene expression was quantified as transcripts per million (TPM) using kallisto128 (v.0.48.0) with 100 bootstraps.
Rachilla hair ploidy measurements
Ploidy assessment was performed on rachillae collected from barley spikes at developmental stage129 approximately Waddington 9.0. Once isolated, rachillae were fixed with 50% ethanol/10% acetic acid for 16âh after which they were stained with 1âµM DAPI in 50âmM phosphate buffer (pH 7.2) supplemented with 0.05% Triton X100. Probes were analysed with a Zeiss LSM780 confocal laser scanning microscope using a Ã20 NA 0.8 objective, zoom Ã4 and image size 512âÃâ512 pixels. DAPI was visualized with a 405ânm laser line in combination with a 405â475ânm bandpass filter. The pinhole was set to ensure the whole nucleus was measured in one scan. Size and fluorescence intensity of the nuclei were measured with ZEN black (ZEISS) software. For data normalization, small, round nuclei of the epidermal proper were used for 2C (diploid) calibration.
Scanning electron microscopy
Sample preparation and recording by scanning electron microscopy were essentially performed as described previously130. In brief, samples were fixed overnight at 4â°C in 50âmM phosphate buffer (pH 7.2) containing 2% v/v glutaraldehyde and 2% v/v formaldehyde. After washing with distilled water and dehydration in an ascending ethanol series, samples were critical-pointâdried in a BalâTec critical-point dryer (Leica Microsystems, https://www.leica-microsystems.com). Dried specimens were attached to carbonâcoated aluminium sample blocks and goldâcoated in an Edwards S150B sputter coater (Edwards High Vacuum, http://www.edwardsvacuum.com). Probes were examined in a Zeiss Gemini30 scanning electron microscope (Carl Zeiss, https://www.zeiss.de) at 5âkV acceleration voltage. Images were digitally recorded.
Linkage mapping of SHORT RACHILLA HAIR 1 (HvSRH1)
Initial linkage mapping was performed using GBS data of a large âMorexâ x âBarkeâ F8 recombinant inbred line (RIL) population47 (European Nucleotide Archive project PRJEB14130). The GBS data of 163 RILs, phenotyped for rachilla hair in the F11 generation, and the two parental genotypes were extracted from the variant matrix using VCFtools122 and filtered as described previously3 for a minimum depth of sequencing to accept heterozygous and homozygous calls of 4 and 6, respectively, a minimum mapping quality score of the SNPs of 30, a minimal fraction of homozygous calls of 30% and a maximum fraction of missing data of 25%. The linkage map was built with the R package ASMap131 using the MSTMap algorithm132 and the Kosambi mapping function, forcing the linkage group to split according to the physical chromosomes. The linkage mapping was done with R/qtl133 using the binary model of the scanone function with the expectation maximization method134. The significance threshold was calculated running 1,000 permutations and the interval was determined by a logarithm of the odds drop of 1. To confirm consistency between the F8 RIL genotypes and F11 RIL phenotypes, three PCR Allele Competitive Extension (PACE) markers were designed through the 3CR Bioscience free assay design service, using polymorphisms between the genome assemblies of the two parents (Supplementary Table 24), and PACE genotyping was performed as described earlier135. To reduce the Srh1 interval, 22 recombinant F8 RILs were sequenced by Illumina WGS, the sequencing reads were mapped on the MorexV3 reference genome sequence assembly9 and the SNP was called. The 100âbp region around the flanking SNPs of the Srh1 interval as well as the sequence of the candidate gene HORVU.MOREX.r3.5HG0492730 were compared with the pangenome assemblies using BLASTN99 to identify the corresponding coordinates and extract the respective intervals for comparison. Gene sequences were aligned with Muscle5 (ref.â136). Structural variation between intervals was assessed with LASTZ116 v.1.04.03. The motif search was carried out with the EMBOSS81 6.5.7 tool fuzznuc.
Cas9-mediated mutagenesis
Guide RNA (gRNA) target motifs in the âGolden Promiseâ HvSrh1 candidate gene HORVU.GOLDEN_PROMISE.PROJ.5HG00440000.1 were selected by using the online tool WU-CRISPR137 to induce translational frameshift mutations by insertion/deletion of nucleotides leading to loss-of-function of the gene. One pair of target motifs (gRNA1a: CCTCGCTGCCCGCCGACGC; gRNA1b: GACAAGACGAAGGCCGCGG) was selected within the HvSrh1 candidate gene on the basis of their position within the first half of the coding sequence and the two-dimensional minimum free energy structures of the cognate single-gRNAs (NNNNNNNNNNNNNNNNNNNNGUUUUAGAGCUAGAAAUAGCAAGUUAAAAUAAGGCUAGUCCGUUAUCAACUUGAAAAAGUGGCACCGAGUCGGUGCUUUU) as modelled by the RNAfold WebServer138 and validated as suggested in ref.â139. gRNA-containing transformation vectors were cloned using the modular CasCADE vector system (https://doi.org/10.15488/13200). gRNA-specific sequences were ordered as DNA oligonucleotides (Supplementary Table 25) with specific overhangs for BsaI-based cloning into the gRNA-module vectors carrying the gRNA scaffold, driven by the Triticum aestivum U6 promoter. Golden Gate assembly of gRNAs and the cas9 module, driven by the Zea mays Polyubiquitin 1 (ZmUbi1) promotor, was performed according to the CasCADE protocol to generate the intermediate vector pHP21. To generate the binary vector pHP22, the gRNA and cas9 expression units were cloned using SfiI into the generic vector140 p6i-2x35S-TE9 which harbours an hpt gene under control of a double-enhanced CaMV35S promoter in its transfer-DNA for plant selection. Agrobacterium-mediated DNA transfer to immature embryos of the spring barley Golden Promise was performed as previously described141. In brief, immature embryos were excised from caryopses 12â14âd after pollination and co-cultivated with Agrobacterium strain AGL1 carrying pHP22 for 48âh. Then, the explants were cultivated for further callus formation under selective conditions using Timentin and hygromycin, which was followed by plant regeneration. The presence of T-DNA in regenerated plantlets was confirmed by hpt– and cas9-specific PCRs (primer sequences in Supplementary Table 25). Primary mutant plants (M1 generation) were identified by PCR amplification of the target region (primer sequences in Supplementary Table 25) followed by Sanger sequencing at LGC Genomics. Double or multiple peaks in the sequence chromatogram starting around the Cas9 cleavage site upstream of the targetâs protospacer-adjacent motif were considered as an indication for chimeric and/or heterozygous mutants. Mutant plants were grown in a glasshouse until the formation of mature grains. M2 plants were grown in a climate chamber under speed breeding conditions (22âh light at 22â°C and 2âh dark at 19â°C, adapted from ref.â142) and genotyped by Sanger sequencing of PCR amplicons as given above. M2 grains were subjected to phenotyping.
FIND-IT library construction
We constructed a FIND-IT library in cv. âEtincelâ (6-row winter malting barley; SECOBRA Recherches) as described in ref.â50. In short, we induced mutations by incubating 2.5âkg of âEtincelâ grain in water overnight at 8â°C following an incubation in 0.3âmM NaN3 at pH 3.0 for 2âh at 20â°C with continuous application of oxygen. After thoroughly washing with water, the grains were air-dried in a fume hood for 48âh. Mutagenized grains were sown in fields in Nørre Aaby, Denmark, and collected in bulk using a Wintersteiger Elite plot combiner. In the following generation, 2.5âkg of grain was sown in fields in Lincoln, New Zealand, and 188 pools of approximately 300 plants each were hand-harvested and threshed. A representative sample, 25% of each pool, was milled (Retsch GM200), and DNA was extracted from 25âg of the flour by LGC Genomics.
FIND-IT screening
The FIND-IT âEtincelâ library was screened as described in ref.â50 using a single assay for the isolation of srh1P63S variant (ID no. CB-FINDit-Hv-014). Forward primer 5â² AATCCTGCAGTCCTTGG 3â², reverse primer 5â² GAGGAGAAGAAGGAGCC 3â², mutant probe 5â²6-FAM/CGTGGACGT/ZEN/CGACG/3âIABkFQ/wild-type probe/5â²SUN/ACGTGGGCG/ZEN/TCGA/3â²IABkFQ/ (Integrated DNA Technologies).
4K SNP chip genotyping
Genotyping, including DNA extraction from freeze-dried leaf material, was conducted by TraitGenetics. srh1P63S mutant, the corresponding wild-type âEtincelâ and srh1 pangenome accessions Morex, RGT Planet, HOR 13942, HOR 9043 and HOR 21599 were genotyped for background confirmation. Pairwise genetic distance of individuals was calculated as the average of their per-locus distances143 using the R package stringdist144 (v.0.9.8). Principal coordinate analysis was done with R120 (v.4.0.2) base function cmdscale on the basis of this genetic distance matrix. The first two principal components were illustrated by ggplot2 (https://ggplot2.tidyverse.org).
Sanger sequencing
gDNA of the srh1P63S variant and âEtincelâ was extracted from 1-week-old seedling leaves (DNeasy, Plant Mini Kit, Qiagen). Genomic DNA fragments for sequencing were amplified by PCR using gene-specific primers (forward primer 5â² TTGCACGATTCAAATGTGGT 3â², reverse primer 5â² TCACCGGGATCTCTCTGAAT 3â²) and Taq DNA Polymerase (NEB) for 35 cycles (initial denaturation at 94â°C/3âmin followed by 35 cycles of 94â°C/45âs, 55â°C/60âs and 72â°C/60âs for extension, with a final extension step of 72â°C/10âmin). PCR products were purified using the NucleoSpin Gel and PCR Clean-Up Kit (Macherey-Nagel) according to the manufacturerâs instructions. Sanger sequencing was done at Eurofins Genomics Germany using a gene-specific sequencing primer (5â² AGAACGGAGAGGAGAGAAAGAAG 3â²).
RNA preparation, sequencing and data analysis
Rachilla tissues from two contrast groups, Morex (short) and Barke (long), and Bowman (long) and BW-NIL-srh1 (short), were used for RNA-seq. The rachilla tissues were collected from the central spikelets of the respective genotypes at rachilla hair initiation (Waddington 8.0) and elongation (Waddington 9.5) stages. Total RNA was extracted using TRIzol reagent (Invitrogen) followed by 2-propanol precipitation. Genomic DNA residues were removed with DNase I (NEB, M0303L). High-throughput paired-end sequencing was conducted at Novogene (Cambridge, UK) with the Illumina NovaSeq 6000 PE150 platform. RNA-seq reads were trimmed for adaptor sequences with Trimmomatic145 (v.0.39) and the MorexV3 genome annotation was used as reference to estimate read abundance with Kallisto128. The raw read counts were normalized to TPM expression levels.
Messenger RNA in situ hybridization
In situ hybridization was conducted in longitudinal sections and cross-sections derived from whole spikelet tissues of Bowman and Morex at rachilla hair elongation developmental stage (Waddington 9.5) with HvSRH1 sense and antisense probes (124âbp). The in situ hybridization was performed as described before146 with few modifications.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.