Data reporting
No statistical methods were used to predetermine sample size. The experiments were not randomized, and the investigators were not blinded to allocation during experiments and outcome assessment.
Materials
Alvimopan, naloxone, mitragynine pseudoindoxyl, DAMGO and loperamide were purchased from Cayman Chemicals and MedChemExpress. Detergents were purchased from Anatrace. The radioligand [3H]naltrexone (specific activity 48.19 Ci per mmol) was generously provided by the National Institute on Drug Abuse Drug Supply Program. All other general laboratory reagents and chemicals were purchased from Millipore Sigma, unless otherwise stated.
Constructs
For recombinant protein expression, full-length human MOR was subcloned into a modified pFastBac1 vector with an N-terminal Flag, 10xHis-tag and thermostabilized b562RIL (ref. 54) along with a C-terminal TwinStrep tag55 and an extra 10xHis-tag. Both N-terminal and C-terminal tags are flanked by HRV-3C protease cleavage sites to allow tag removal. For the G-protein-free ‘inactive’ MOR structure, two point mutations M264L and K2696.24R in ICL3 were incorporated, to allow Nb6 binding56.
For all BRET assays, transient transfections of wild-type full-length human MOR were performed using the pcDNA 3.1(−) vector in human embryonic kidney 293F (HEK293F) mammalian cells. These receptor constructs were expressed under the cytomegalovirus promoter and included an N-terminal haemagglutinin signal peptide followed by a Flag tag. For TRUPATH24 and GloSensor assays, the MOR construct was untagged and contained no fusion elements. In the RG-BRET assay, Rluc8 was fused immediately after the C terminus of the wild-type MOR construct.
In TRUPATH assays24, a tricistronic vector encoding Gαi1, Gβ1 and Gγ2 was used, in which GFP2 was fused to the N terminus of Gγ2 and Rluc8 was inserted after residue G90 of the Gαi1 subunit23. For RG-BRET assays, a Gαi1β1γ2 construct was used with GFP2 fused to the N terminus of Gγ2. The pOZITX-S1 plasmid, used as a control in these assays, was obtained from J. Javitch (Addgene plasmid no. 184925).
Cell culture and transfections
HEK293F cells were cultured in FreeStyle293 Expression Medium (Gibco) at 37 °C with 5% CO2 and shaking at 110 rpm. For transfections, cells were seeded 1 day before and transfected at a cell density of 1.0 × 106 cells per ml using polyethyleneimine (PEI) with a DNA:PEI ratio of 1:2. For RG-BRET assays, MOR–Rluc8 and Gαi/Gβ1/Gγ2–GFP2 constructs were transfected at a 1:2 ratio. TRUPATH BRET assays used MOR and Gαi1–Rluc8/Gβ1/Gγ2–GFP2 transfected at a 1:1 ratio. When pOZITX-S1 was used, Gαi/Gβ1/Gγ2–GFP2 and pOZITX-S1 plasmids were transfected at a 1:1 ratio. For MOR–Gαi-mediated cAMP inhibition assays, cells were cotransfected with wild-type human MOR, along with a split-luciferase-based cAMP biosensor (GloSensor, Promega) at a 1:1 ratio.
BRET2 assays
For experiments with intact cells, cells were washed with assay buffer (20 mM HEPES pH 7.5, 1× HBSS) and transferred to white-bottom 96-well plates. For permeabilized cell assays, collected cells were washed twice with permeabilization buffer KPS (140 mM KCl, 10 mM NaCl, 1 mM MgCl2, 0.1 mM KEGTA and 20 mM NaHEPES, pH 7.2). Cells were permeabilized using 10 μg ml−1 high-purity digitonin (GoldBio) and treated with apyrase (2 U ml−1, Sigma-Aldrich), optionally with increasing GDPβS concentrations, then transferred to 96-well plates containing the respective ligand in ligand buffer (20 mM HEPES pH 7.5, 1× HBSS, 0.1% BSA) or KPS for permeabilized cells.
Steady state BRET measurements were performed using a PHERAstar FSX multimode plate reader (BMG Biotech). BRET2-specific filters were used: emission 410 nm (80 nm slit) and emission 515 nm (30 nm slit). Coelenterazine 400a (5 μM; Nanolight) dissolved in NanoFuel Solvent was added to all wells immediately before measuring with Rluc8. Raw netBRET signals were calculated as the emission intensity at 515 nm divided by the emission intensity at 410 nm, with no normalization or further data treatment applied to reported results.
Each assay was conducted in at least three biologically independent (separate transfection) experiments, on different days, with all data points measured in triplicate. EC50 and half-maximal inhibitory concentration (IC50) values were calculated in GraphPad Prism 10 (v.10.6.0) by fitting the raw netBRET results from each experiment, followed by non-linear regression using the equations: y = Bottom + (Top − Bottom)/(\(1+{10}^{({\mbox{logEC}}_{50}-x)}\)) and y = Bottom + (Top − Bottom)/(\(1+{10}^{(x-{\mbox{logIC}}_{50})}\)).
cAMP inhibition assays
At 48 h post-transfection, cells were washed with assay buffer (20 mM HEPES, 1× HBSS, pH 7.4) and plated into 96-well white cell culture plates at a density of 200,000 cells in 90 μl per well. Ligands were prepared as 3× solutions in assay buffer (20 mM HEPES, 1× HBSS, pH 7.4). Media was aspirated and cells were incubated with 30 μl per well of drug buffer (20 mM HEPES, 1× HBSS, pH 7.4), followed by addition of 30 μl of 3× drug solutions for 15 min in the dark at room temperature. Cells then received 30 μl of luciferin (Goldbio, 4 mM final concentration) supplemented with isoproterenol (100 μM final concentration) to stimulate production of endogenous cAMP through β2 adrenergic Gs activation, and were incubated in the dark at room temperature. After 15 min, luminescence intensity was quantified using a PHERAstar FSX multimode plate reader (BMG Biotech). Data were plotted as a function of ligand or nucleotide concentration and analysed using log (ligand or nucleotide) versus response in GraphPad Prism (v.10.6.0).
Radioligand saturation binding assays
Radioligand binding assays were conducted using membrane fractions from HEK293F cells transiently expressing human wild-type MOR. For membrane preparation, cell pellets expressing human wild-type MOR and Gαi1-Gβ1-Gγ2 at a 1:1 ratio were gathered and resuspended in hypotonic buffer (10 mM HEPES pH 7.5, 10 mM MgCl2, 20 mM KCl supplemented with 2 mM AEBSF, 14 μM E-64, 1 μM leupeptin and 0.3 μM aprotinin). Cells were dounce homogenized and centrifuged at 175,000g in two rounds to obtain membrane fractions. Membrane protein concentration was determined to be roughly 4 mg ml−1 using a Bradford assay (Pierce). Aliquots were flash frozen in liquid nitrogen and stored at −80 °C until use.
Competition binding assays were setup in 96-well plates containing membrane fractions diluted to 0.15 mg ml−1, along with [3H]naltrexone at 2 nM and a ‘cold’ naltrexone dose (100 µM–10 pM), all prepared in binding buffer (10 mM HEPES, 10 mM MgCl2, 20 mM KCl, 0.1% BSA and 100 µM Bacitracin). Competition reactions were incubated for 1 h and terminated by vacuum filtration onto cold 0.3% PEI-soaked GF/A filters, followed by 3 rounds of washing with cold 50 mM HEPES (pH 7.50). Counts were read using a Microbeta2 plate reader (PerkinElmer) for 1 min per well. Results were analysed in GraphPad Prism v.10.6.0, and the inhibitor constant (Ki) was determined using the ‘One site – Fit Ki’ model equation: logEC50 = log(\({10}^{\mbox{log}{K}_{i}\times (1\,+\,\mbox{RadioligandNM}/\mbox{HotKdNM})}\)), y = Bottom + (Top − Bottom)/(\(1+{10}^{(x-{\mbox{logEC}}_{50})}\)).
MOR expression and purification
Recombinant expression of the pFastBac1-MOR constructs was carried out in Spodoptera frugiperda (Sf9) insect cells using the Bac-to-Bac expression system (Gibco). Recombinant baculovirus was added at a multiplicity of infection of 5 to Sf9 cells cultured at a density of 3–3.5 × 106 cells per ml in ESF921 medium (Expression Systems) supplemented with 1% (v/v) production boost additive. To enhance receptor surface expression and improve protein yield, 10 µM naloxone was added during protein expression. Cells were incubated for 48 h at 27 °C with shaking at 100 rpm, then collected by centrifugation, washed with PBS and stored at −80 °C until further use.
For membrane preparation, frozen cell pellets were thawed on ice and resuspended in hypotonic buffer (10 mM HEPES pH 7.5, 10 mM MgCl2, 20 mM KCl, with protease inhibitors: 2 mM AEBSF, 14 μM E-64, 1 μM leupeptin and 0.3 μM aprotinin). The suspension was homogenized using a glass dounce homogenizer and further treated with hypertonic buffer (hypotonic buffer + 1 M NaCl). Membranes were harvested by ultracentrifugation at 150,000g for 45 min at 4 °C. This washing and centrifugation process was repeated 2 more times, with 10 μM naloxone included in the final 2 washes. Final membrane pellets were homogenized in purification buffer (40 mM HEPES pH 7.5, 150 mM NaCl and 10 μM naloxone) supplemented with 20% glycerol and stored at −80 °C.
For protein solubilization, membranes were thawed in the presence of 2 mg ml−1 iodoacetamide and diluted in 2× solubilization buffer (40 mM HEPES pH 7.5, 150 mM NaCl, 1.0% w/v lauryl maltose neopentyl glycol (LMNG) and 0.1% w/v cholesteryl hemisuccinate (CHS)) and incubated for 8 h at 4 °C. Insoluble debris was removed by centrifugation at 150,000g for 1 h at 4 °C. The supernatant was incubated with M2 anti-Flag affinity resin (Sigma) for 2 h at 4 °C. The resin was washed with 20 column volumes of wash buffer (40 mM HEPES pH 7.5, 150 mM NaCl, 0.001% LMNG, 0.0001% CHS and 10 μM naloxone). Protein was eluted using the same buffer supplemented with 200 μg ml−1 DYKDDDDK peptide (GenScript). The eluate was concentrated and used for complex formation with Nb6M or heterotrimeric G proteins.
Expression and purification of Nb6M
Wk6 Escherichia coli (American Type Culture Collection) were transformed by heat shock with the Nb6 plasmid56,57 and grown overnight in starter cultures containing 100 μg ml−1 ampicillin. These cultures were used to inoculate 2 l of Terrific Broth supplemented with 100 μg ml−1 ampicillin and grown at 37 °C with shaking until log phase (optical density at 600 nm (OD600) between 0.5 and 1) was reached. Protein expression was induced with 1 mM isopropyl-β-d-thiogalactoside (IPTG), and cultures were incubated overnight at 28 °C. Cell pellets were gathered by centrifugation, washed with PBS and flash frozen in liquid nitrogen.
For purification, Nb6-containing pellets were thawed and resuspended in TES buffer (50 mM Tris pH 8.0, 0.5 mM EDTA, 20% w/v sucrose) at 15 ml per litre of culture, supplemented with protease inhibitor cocktail and incubated on an orbital shaker at 4 °C for 1 h. An extra 30 ml per litre of 1:4 diluted TES was added, followed by another 45 min of incubation at 4 °C. Cell debris was removed by ultracentrifugation at 100,000g for 30 min and the clarified supernatant was passed through a 0.45-µm filter. The filtered solution was supplemented with 20 mM imidazole and loaded onto a Ni-NTA affinity column prechilled to 4 °C.
The column was washed with 10 column volumes of wash buffer (250 mM NaCl, 50 mM Tris pH 7.5, 10 mM imidazole) and Nb6M was eluted with buffer containing 250 mM imidazole. Fractions were pooled, concentrated, supplemented with 10% glycerol and snap-frozen in liquid nitrogen.
Expression and purification of NabFab
Chemically competent E. coli C43 cells were transformed by heat shock with the NabFab plasmid58. A starter culture in Terrific Broth medium was inoculated and grown overnight at 37 °C with shaking. These cultures were used to inoculate 2 l of Terrific Broth supplemented with 100 μg ml−1 ampicillin and grown at 37 °C with shaking until log phase (OD600 between 0.5 and 1) was reached. Protein expression was induced with 1 mM IPTG, and cultures were incubated overnight at 28 °C. Cell pellets were collected by centrifugation, washed with PBS and flash frozen in liquid nitrogen.
For purification, thawed cell pellets were resuspended in lysis buffer (20 mM sodium phosphate pH 7.4, 150 mM NaCl, DNaseI, and protease inhibitor cocktail) and lysed by sonication. The lysate was heat-treated at 63–65 °C for 30 min and clarified by centrifugation at 20,000g for 30 min. The supernatant was loaded onto a Protein G column pre-equilibrated with 20 mM sodium phosphate, 500 mM NaCl, pH 7.4. NabFab was eluted with 0.1 M acetic acid and directly applied to a Resource S cation exchange column equilibrated in buffer A (50 mM sodium acetate, pH 5.0). The column was washed with five column volumes of buffer A, and NabFab was eluted using a 0–100% gradient of buffer B (50 mM sodium acetate, 2 M NaCl, pH 5.0). Eluted protein was dialysed overnight into 150 mM NaCl, 20 mM HEPES, pH 7.5 and concentrated to 3 mg ml−1 for complex formation.
Expression and purification of G proteins and scFv16
Wild-type Gαi1 was co-expressed with Gβ1 and γ2 subunits in Sf9 insect cells using the Bac-to-Bac system in ESF921 medium (Expression Systems). Cells were seeded at a density of 2 × 106 cells per ml and infected with P1 baculovirus at a multiplicity of infection ratio of 10:5 (Gαi1:Gβγ). After 48 h of incubation at 27 °C with shaking at 100 rpm, cells were gathered by centrifugation, washed with ice-cold PBS and stored at −80 °C until use. Gαi1βγ heterotrimers were purified using established protocols59,60. Frozen pellets were thawed and homogenized in hypotonic buffer containing 10 µM GDP and 5 mM β-mercaptoethanol, followed by centrifugation at 100,000g. The resulting pellet was solubilized for 90 min at 4 °C in a buffer containing 20 mM HEPES pH 7.5, 100 mM NaCl, 1% sodium cholate, 0.05% DDM, 5 mM MgCl2, 5 mM β-mercaptoethanol, 15 mM imidazole, 10 µM GDP and protease inhibitors. Insoluble debris was removed by centrifugation at 150,000g for 45 min at 4 °C. The supernatant was loaded onto Ni-NTA resin and eluted with buffer containing 300 mM imidazole. The protein was concentrated using a 50-kDa cutoff centrifugal concentrator (Amicon) and further purified through anion exchange chromatography using a 1 ml HiTrap Q FF column (Cytiva).
The scFv16 single-chain antibody fragment was expressed in Sf9 cells at a density of 2 × 106 cells per ml with baculovirus infection at a multiplicity of infection of 5. After 72 h, culture media was collected by centrifugation at 1,000g for 15 min at 4 °C. The pH of the media was adjusted to 7.5 using 1 M Tris, followed by the addition of 1 mM NiCl2 and 5 mM CaCl2 to precipitate chelators. The precipitate was removed by ultracentrifugation at 100,000g. The clarified supernatant was incubated with Ni-NTA resin for 3 h, washed with buffer containing 20 mM HEPES pH 7.5, 100 mM NaCl, 15 mM imidazole, 0.00075% LMNG and 0.000075% CHS, and eluted with the same buffer supplemented with 300 mM imidazole. The purified scFv16 was concentrated to roughly 10 mg ml−1 and stored at −80 °C.
Complexation of MOR and G protein heterotrimers
Purified MOR–naloxone was incubated with Gαiβγ heterotrimer at a 1:1.2 molar ratio for 1 h at room temperature. Apyrase was then added to catalyse hydrolysis of GDP (for ‘re-bound GDP’ and nucleotide-free specimen). For the nucleotide-free MOR structure, scFv16 was added at 1.2 molar excess. For the ‘constant GDP’ samples, the specimen was not treated with apyrase, and instead supplemented with 200 μM (naloxone) or 500 μM (loperamide) GDP in all subsequent purification buffers. The mixture was incubated at room temperature for 90 min. The complex was subsequently purified with an extra round of Flag resin, to separate from excess G protein by size-exclusion chromatography using a Superdex 200 10/300 column equilibrated with buffer containing 40 mM HEPES pH 7.5, 100 mM NaCl, 10 μM naloxone, 0.00075% LMNG and 0.000075% CHS. Peak fractions were pooled, concentrated and immediately used for cryoEM studies. For the inactive MOR structure, purified MOR was incubated with Nb6M and NabFab in molar excess of 2:1, incubated on ice overnight and finally purified by size-exclusion chromatography, with identical conditions described above.
CryoEM sample preparation, data collection and 3D reconstruction
MOR–Nb6–naloxone, MOR–Gi–naloxone, complexes in LMNG or CHS micelles were used immediately after concentrating the monomeric size-exclusion chromatography peak to 1–2 mg ml−1 using a 50-kDa cutoff Amicon concentrator. For cryoEM grid preparation, 3 µl of purified protein complex was applied to freshly glow-discharged UltrAuFoil 1.2/1.3 300 mesh grids (Quantifoil), blotted for 2.5–4 s at 95% relative humidity and 4 °C, then vitrified in liquid ethane using a Vitrobot Mark IV (Thermo Fisher). For the ‘re-bound GDP’ samples, the purified specimen was incubated with 100 µM GDPβS for 1 h, before grid freezing. For the ‘constant GDP’ samples, the purified specimen was further supplemented with 500 μM GDP (naloxone) and 1 mM GDP (loperamide) and incubated before grid freezing.
Micrographs were collected using an aberration-free image shift data collection scheme (four images per hole) with EPU data acquisition software (version 2.0) on a Titan Krios microscope (Thermo Fisher) operating at 300 keV. The microscope was equipped with a K3 direct-electron detector and post-BioQuantum GIF energy filter using a 20 eV slit size (Gatan). Images were collected with a total exposure time of 1.8 s, total dose of 55–60 e−/Å2 and defocus ranging from −1 µm to −3 µm.
Single particle cryoEM image processing
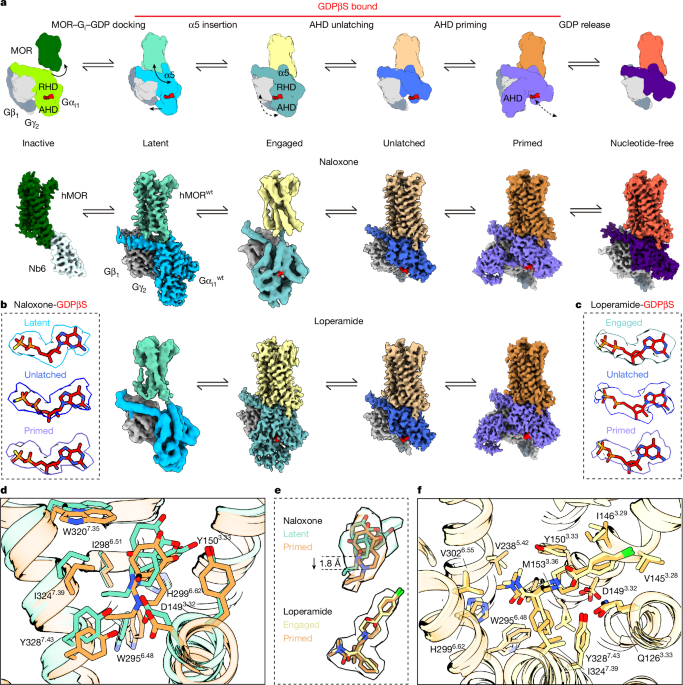
Motion correction of micrograph videos was carried out using MotionCor2 (ref. 61). All subsequent data processing steps were performed using the software package cryoSPARC62 (v.4.7.1; Structura Biotechnology) (Fig. 1, Extended Data Figs. 3–6 and Supplementary Tables 1–3), using established protocols. For naloxone-bound complexes, a total of 30,264 (MOR–Nb6), 45,722 (‘re-bound’ MOR–Gi–GDP), 17,742 (‘constant’ MOR–Gi–GDP) and 32,514 (MOR–Gi–nucleotide-free) micrographs were preprocessed through patch contrast transfer function estimation (default parameters), and micrographs with average contrast transfer function resolution estimates above 4 Å were discarded. For loperamide-bound complexes, 22,868 (re-bound MOR–Gi–GDP) and 22,146 (constant MOR–Gi–GDP) micrographs were collected and preprocessed similarly. An initial round of processing was carried out on a subset of the data (roughly 5,000 micrographs), using the reference-free Blob picker (140 Å particle diameter) routine, followed by two-dimensional (2D) classification until 2D classes with clearly distinguishable transmembrane domain (TMD) densities were obtained. Ab initio reconstruction of these 2D classes was performed to obtain a 3D model used for template-based picking and 3D reconstructions of all three full datasets (Extended Data Figs. 3–5). Particle picking on the entire dataset (20 Å reference low pass) was followed by extraction with a box size of 128 pixels (bin 4; 512 pixels uncropped box size). Three to four rounds of 2D classification were performed to obtain homogenous 2D classes with distinguishable TMD densities (Extended Data Figs. 3–5), after which particles were re-extracted (bin 2, box size 256). Further 2D classification was used to separate complexes with ‘open’ or ‘closed’ AHD conformations, followed by multiclass ab initio reconstructions of each particle subset. The resulting 3D volumes were used to perform several rounds of heterogeneous refinement, on the re-extracted particle stack resulting in segregation of particles into five classes. A combination of ab initio reconstruction, non-uniform refinement and 2D classification was performed on particle subsets corresponding to MOR–Gi complexes. Finally, local refinements with a manually created mask around the TMD, masking the micelle and the heterotrimeric G protein yielded the final reconstructions of maps ranging between 2.8 Å and 3.8 Å. The final ‘focused’ maps were merged in Chimera for subsequent model building and refinement.
Model building and refinement
Refinements were based on PDB ID 1GP2 (Gαi1 protein heterotrimer, GDP-bound)40, PDB 7UL4 (MOR–Nb6-alvimopan)56 and PDB 8EF6 (MOR–Gi-DN-morphine)47, for which residues in the sequence were reverted to the respective wild-type Gαi. The ordered AHD conformation for the ‘primed’ state was modelled after rhodopsin PDB ID 6CMO (ref. 63). The models were fitted into the density map in UCSF Chimera (version 1.17.3)64 and manually adjusted to fit the density map in COOT (version 0.9.2)65. Subsequently, the generated model was automatically refined in phenix.real_space_refine66,67 (version 1.21.2) and manually adjusted in COOT (version 0.9.2)65, for several iterations. The final geometry validation statistics including clashscore and Ramachandran analysis were calculated by MolProbity68. The final refinement statistics were generated using the ‘comprehensive validation (cryoEM)’ function in phenix (version 1.21.2)67.
MD simulations
For our MD simulation, we deployed GROMACS v.2024.5 (ref. 69) using CHARMM36 all-atom force-field parameters and topologies70,71. Ligand force-field parameters and topologies for naloxone and loperamide were generated using the Ligand Reader & Modeler tool available through the CHARMM-GUI webserver. The starting conformations of these complexes were obtained from the corresponding cryoEM structures determined in this study. MOR, Gi and MOR–Gαi heterotrimers (GDP) in the respective state were embedded in a lipid bilayer consisting of dipalmitoylphosphatidylcholine (DPPC), dioleoylphosphatidylcholine (DOPC) and cholesterol with ratio DPPC:DOPC:CHL 0.55:0.15:0.30, referencing simulations performed on GPCRs72. The missing Gαi1 AHD in the unlatched structure was manually modelled using the latent and primed structures as a template, placing the AHD roughly at its halfway point, with structure regularization and torsion angle optimization carried out using phenix. The N-terminal Gly of Gα was myristoylated, while Cys3 of the same chain was palmitoylated. In Gγ, the C terminus was capped using N-methylamide, as well as geranylgeranylation of Cys68. The palmitoyl group was also added to Cys172 of MOR. Initial membrane coordinates were assigned by the Positioning of Proteins in Membranes server through the CHARMM-GUI interface73. Systems were solvated in TIP3 water molecules, and Na+, Cl−, Mg2+ ions were placed in the systems to obtain 100 mM NaCl and 10 mM MgCl2. The box size was determined on the basis of the protein extent of each model (xy from 80 Å for the inactive MOR–naloxone to 120 Å for MOR–Gαi1 complex, z 160 Å).
All systems underwent initial energy minimization for 50,000 steps using the steepest descent algorithm and a 100 kJ mol−1 nm−1 threshold, followed by equilibration simulations for a total time of 10 ns. The equilibration was performed in the NVT ensemble followed by the NPT ensemble for 6 steps (time step of 1–2 fs) with V-rescale thermostat at 303.15 K and Parrinello–Rahman barostat at 1 atm. Five separate replicates of production runs were subsequently performed for 1 µs each under NPT ensemble with Parrinello–Rahman barostat at 1 atm and V-rescale thermostat at 303.15 K, with random assignment of velocities. Simulations were executed on the graphical processing unit clusters at the Center for Advanced Computing of the University of Southern California. MD trajectory analysis was carried out using the GROMACS analysis toolkit, the MDTraj software package74 and MDCiao75.
Data statistical analysis
For BRET2 nucleotide competition results, log transformed EC50 values were analysed using one-way analysis of variance within each drug group. For comparisons between only two groups, unpaired two-tailed Student’s t-tests were performed to compare each condition. A significance threshold of α = 0.05 was applied for both one-way analysis of variance and Student’s t-test analyses. Statistical significance is denoted by asterisks: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; NS indicates not significant. EC50 values are reported as the mean ± s.d. across three independent replicates.
Figures and graphical illustrations
PyMol v.3.1.6.1 (ref. 76) (Schrödinger), UCSF Chimera v.1.17.3 (ref. 64), UCSF ChimeraX v.1.9 (ref. 77), ChemDraw Professional v.22.2.0 (PerkinElmer), GraphPad Prism v.10.6.0 and Adobe Illustrator 2021 v.29.7.1 were used to create all illustrations and figures. All reported root mean-square deviation values were calculated using the align command in PyMol, with either global alignment of the receptor or Gαi RHD. All netBRET data are plotted as ratios of the raw emission data, without baseline correction, normalization or any other data treatment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.