Antibodies, plasmids and reagents
Western blot analyses were performed using primary antibodies at a dilution of 1:1,000 unless otherwise specified. Anti-STAT1 (D1K9Y, 14994), anti-STAT2 (D9J7L, 72604), anti-STAT3 (Rabbit, 79D7, 4904; Mouse, 124H6, 9139), anti-STAT4 (C46B10, 2653), anti-STAT5 (D2O6Y, 94205), anti-STAT6 (D3H4, 5397), anti-JAK1 (6G4, 3344), anti-JAK2 (D2E12, 3230), anti-JAK3 (D7B12, 8863), anti-TYK2 (E9H4T, 35615), anti-p65 (D14E12, 8242), anti-CD80 (E6J6N, 54521), anti-CD86 (E5W6H, 19589), anti-IL-6Rα (E7H4J, 39837), anti-GP130 (3732), anti-pSTAT1 (58D6, 9167), anti-pSTAT3 (D3A7, 9145), anti-pSTAT5 (D47E7, 4322), anti-pSTAT6 (D8S9Y, 56554), anti-pJAK1 (D7N4Z, 74129), anti-pJAK2 (C80C3, 3776), anti-pJAK3 (D44E3, 5031), anti-pp65 (93H1, 3033) and anti-β-actin (4967) were from Cell Signaling Technology. Anti-JAK2 (C-10, sc-390539), anti-GMRβ (F-12, sc-393281) and anti-β-actin (C4, sc-47778) were from Santa Cruz Biotechnology. Anti-pTYK2 (PA5-37762) was from Invitrogen. HRP horse anti-mouse IgG antibody (PI-2000, 1:5,000) and HRP goat anti-rabbit IgG (PI-1000, 1:5,000) were from Vector Laboratories.
For the in vivo experiments, anti-mouse CD8 (clone YTS 169.4, BE0117), anti-mouse PD-L1 (clone 10F.9G2, BE0101) and anti-IgG2b (clone LTF-2, BE0090) were from BioXcell. SD-36 and SD-2301 were designed and synthesized in our laboratory. FLLL32 (JAK2 inhibitor X, 5301530001) and STAT5 inhibitor (573108) were purchased from Sigma-Aldrich. Plasmids expressing shRNAs targeting Stat3 (TRCN0000071453, TRCN0000301946), Stat1 (TRCN0000054924), Jak2 (TRCN0000023649, TRCN0000023651, TRCN0000023652) and Gmrβ (TRCN0000067025, TRCN000067026) were from Sigma-Aldrich.
Cell culture
JAWSII, 293T, B16F10, CT26, EMT6, LLC and 4T1 cells were purchased from the American Type Culture Collection (ATCC). MC38 mouse colon cancer cell line was obtained from the University of Texas Southwestern Medical Center (Y.-X. Fu). Ovarian cancer cell line luciferase-ID8 cells were previously reported24. All cell lines were tested for mycoplasma contamination by MycoAlert Mycoplasma Detection kit and confirmed negative for Mycoplasma.
The 293T cells were transfected with packaging plasmids and non-targeting lentivirus vector, or a lentivirus vector encoding shRNAs targeting Stat3, Stat1, Jak2 or Gmrβ. The virus-containing supernatant was collected 48 h after transfection. JAWSII cells were transfected with the lentivirus. Then the cell lysates were analysed by immunoblotting.
Bone marrow-derived dendritic cells
Bone marrow was collected from the femurs and tibias of mice. Red blood cells were lysed using ACK lysis buffer. Bone marrow-derived DCs (BMDCs) were generated with GM-CSF (20 ng ml−1) and FLT3 ligand (100 ng ml−1) in IMDM (Iscove’s Modified Dulbecco’s Medium; Gibco, 12440-053) supplemented with 10% FBS, 1% penicillin-streptomycin and 55 μΜ β-mercaptoethanol for 7–10 days. BMDCs were either sorted as cDC1s (CD11c+XCR1+) using a FACSAria flow cytometry sorter (BD Biosciences) or purified using biotin anti-mouse XCR1 antibody (BioLegend,148212) and anti-biotin microbeads (Miltenyi Biotec, 130-090-485) following the manufacturer’s protocols for subsequent experiments. Primary cell cultures were tested to be mycoplasma-free by MycoAlert Mycoplasma Detection kit.
ELISA
Culture media were collected from Stat3+/+ and Stat3−/− cDC1s and centrifuged at 8,000g for 5 min. GM-CSF (MGM00) and IL-6 (DY406) were detected in the culture supernatants using the ELISA kit.
CD8 depletion
CD8+ T cells were depleted with anti-CD8 (YTS 169.4, BioXCell) antibodies. Anti-CD8 (100 μg per mouse) antibodies were injected intraperitoneally at the beginning of tumour inoculation and continuously administered every three days.
Drug cellular uptake
DCs and LLC tumour cells were treated with 1 μM SD-36 for 12 h. Then, DCs and LLC (106 each) were centrifuged at 1,500 rpm for 5 min at 4 °C and washed 3 times with ice-cold PBS. The pellets were resuspended in 100 μl of a 50% (v/v) water/methanol solution and subjected to 3 freeze–thaw cycles. After the final thaw cycle, these samples were centrifuged at 14,000 rpm, 4 °C for 20 min, and the supernatant were collected. All samples were submitted to the pharmacokinetics core (University of Michigan) for analysis.
Immunoprecipitation and immunoblot analysis
Cells were lysed in immunoprecipitation lysis buffer (50 mM Tris-HCl pH 7.4, 120 mM NaCl, 1 mM EDTA and 0.5% NP-40) and supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific). Cells were repeatedly passed through a 21-gauge needle with sonication. Then 1,000 µg total cell lysates were incubated with the appropriate antibody (2 µg), with rotation overnight at 4 °C, followed by a 3-h incubation with Protein A/G Sepharose beads (Santa Cruz Biotechnology). Immune complexes were washed three times with wash buffer (20 mM Tris-HCl pH 7.4, 100 mM NaCl, 1 mM EDTA and 0.2% NP-40); then the immunoprecipitated proteins were denatured by the addition of sample buffer (Bio-Rad) and boiled for 10 min, resolved by SDS–PAGE, and immunoblotted with indicated antibodies.
Cells were lysed in RIPA buffer (Thermo Fisher Scientific) supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific). The protein concentrations of cell lysates were determined by BCA protein assay kit (Thermo Fisher Scientific). Equivalent amounts of total cellular protein were separated by SDS–PAGE, transferred to polyvinylidene fluoride membranes, and immunoblotted with the indicated antibodies.
For phospho-antibody array cDC1s were treated with LPS (20 ng ml−1) for 3 h and lysed for protein extraction. Lysates were analysed using the Proteome Profiler Phospho-Kinase Array Kit (R&D Systems, number ARY003C) according to the manufacturer’s instructions. Western blotting data were processed and analysed by image lab 6.1 (Bio-Rad) and ImageJ 1.51n (NIH).
Immunohistochemistry staining
For histological analysis, tissue samples were collected, fixed in 10% formalin (Sigma) and processed for formalin-fixed paraffin-embedded tissue analysis. Four-micrometre paraffin sections underwent heat-induced epitope retrieval. Staining was performed with anti-mouse CD31 antibody (Cell Signaling Technology, D8V9E, 77699, 1:100) using an automated immunostainer (Biocare Intellipath, Biocare Medical). Sections were counterstained with haematoxylin (Biocare Medical), and slides were scanned with the Aperio AT2 Scanner (Leica Biosystems Imaging). Quantification was performed with QuPath v0.5.1.
Quantitative PCR
Total RNA was isolated from cells by column purification (RNeasy Micro Kit, Qiagen) with DNase treatment. cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) with random hexamer primers. Quantitative PCR was performed on cDNA using Fast SYBR Green Master Mix (Thermo Fisher Scientific) on a QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific). Gene expression was quantified using specific primers (Extended Data Table 3). Fold changes in messenger RNA expression were calculated with the ΔΔCt method using Actb as an endogenous control. The results are expressed as fold changes normalized to the controls.
Synthesis of SD-2301 and its chemical data
The synthesis of SD-2301 is outlined in scheme 1 (Supplementary Fig. 1). It began with a known compound, 1, which was converted into compound 2 through a two-step process. First, compound 1 was reacted with hept-6-ynoic acid to form an amide, which was then saponified using LiOH in aqueous media to yield acid intermediate 2 in 65% overall yield. Azide 6 was synthesized in 80% yield from commercially available amine 5 using a previously reported method. A click reaction between alkyne acid 2 and azide 6, with sodium ascorbate and CuSO4, resulted in acid intermediate 3 in 78% yield. Amine 7 was prepared from Boc-l-glutamine through a two-step procedure. Initially, Boc-l-glutamine was coupled with substituted benzyl amine in the presence of HATU (hexafluorophosphate azabenzotriazole tetramethyl uronium) and DIPEA (N,N-diisopropylethylamine) in DMF (N,N-dimethylformamide) to form a Boc-protected amide, which was then Boc-deprotected using 4 (N) HCl in DCM to give amine 7 in 70% yield over two steps. The coupling of acid 3 with amine 7 in the presence of HATU and DIPEA in DMF produced a Boc-protected intermediate. This intermediate was then Boc-deprotected using 4 (N) HCl in dioxane to yield amine intermediate 4 in 75% yield over two steps. Finally, compound 4 was converted into SD-2301 in 86% yield through an amide formation reaction with literature known intermediate 8, using HOBt and DIPEA in DMF.
The purity of SD-2301 was confirmed by ultra-performance liquid chromatography (UPLC) to be >99%. UPLC−MS (ESI) m/z: calculated, 683.7 for C64H80N13O15PS2 [M + 2H]+/2; found, 684.09 (Supplementary Fig. 2). Proton nuclear magnetic resonance (1H-NMR) and carbon nuclear magnetic resonance (13C-NMR) spectroscopies were performed on Bruker Advance 400 NMR spectrometers, and chemical shifts are reported in parts per million (ppm) relative to an internal standard (Supplementary Figs. 3 and 4).
In vivo mouse experiments
Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Michigan. All mice were maintained under specific pathogen-free housing (about 22 °C with approximately 40% humidity) on a 12-h dark:12-h light cycle. The following mice (at 6–8 weeks of age) (The Jackson laboratory) were used for this study: C57BL/6J, BALB/cJ, NOD-scid IL2Rγ null (NSG), Rag1tm1Mom (Rag1–/–), C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I), B6.129S(C)-Batf3tm1Kmm/J (Batf3−/−), B6.129S(Cg)-Stat1tm1Dlv/J (Stat1−/−), B6(129S4)-Xcr1tm1.1(cre) Kmm/J (Xcr1cre), B6.129S1-Stat3tm1Xyfu/J (Stat3fl/fl) mice and CD-1 mice were obtained from Charles River Laboratories. Stat5b–/– C57BL/6J mice were from the National Institutes of Health (Warren Leonard). Stat3fl/fl mice were crossed with Xcr1cre mice to obtain specific STAT3 deficiency in DC1s (Stat3−/− mice). In-house littermates were used in the control arm when specific mouse strains were generated in-house.
MC38 (2 × 106), CT26 (105), EMT6 (105), B16F10 (2 × 105) and LLC (2 × 105) cells were injected subcutaneously into syngeneic mice. Luciferase-expressing ID8 cells (2 × 106) were injected into the peritoneal cavity of female mice. Luciferase-expressing 4T1 cells (105) were injected into female mouse mammary fat pad. From day 5–7, SD-36 (low doses: 10 or 20 mg kg−1; high dose: 100 mg kg−1) or SD-2301 (5 mg kg−1) was administered intravenously every 3 days. In 4T1 tumour metastasis model, treatment with SD-36 (20 mg kg−1) started on day 14, followed by every 3 days. In some cases, peritoneal tumour-bearing mice were similarly treated with SD-36 (20 mg kg−1) or SD-2301 (5 mg kg−1) or were injected intraperitoneally with FLLL32 (30 mg kg−1) for 24 h. For the ICB experiments, PD-L1 monoclonal antibody or isotype antibody (BioXcell, 200 μg) were administrated intraperitoneally into tumour-bearing mice, starting on day 3, then repeated this treatment every 3 days. For DC1 transfusion experiments, bone marrow-derived cDC1s (2 × 106) were sorted and injected intravenously into tumour-bearing Batf3−/− mice on day −2 and day 8. SD-36 (20 mg kg−1) was administered intravenously every three days, starting on day 5. Tumour inoculation time was considered day 0. Tumour cells were inoculated into age-, sex- and source-matched mice. Tumour size was measured using calipers with a Vernier scale and calculated as previously46.
To evaluate tolerability and toxicity, tumour-bearing C57BL/6J mice received intravenous injections of SD-2301 (5 mg kg−1) every 3 days for 18 days, with body weights were monitored throughout.
For pharmacokinetic evaluation, female CD-1 mice were administered a single intravenous dose of SD-2301 at 5 mg kg−1 with 25% of PCP as the dosing vehicle. Then, 250–300 μl blood samples were collected from 5 min to 24 h. Following centrifugation at 15,000 rpm for 10 min, at least 100 μl of plasma was collected. All samples were analysed by the Pharmacokinetic and Mass Spectrometry core at the University of Michigan. Compound concentrations in plasma were determined using a validated LC-MS/MS method with an internal control. Chromatographic separation was achieved with a Waters XBridge-C18 column (5 cm × 2.1 mm, 3.5 μm) on a Shimadzu HPLC system. Detection was performed on an AB Sciex QTrap 5500 mass spectrometer in positive-ion MRM mode. The mobile phases were 0.1% formic acid in water (A) and in acetonitrile (B). The gradient for B was: 10% (0–0.3 min), increased to 95% at 0.7 min, held for 2.3 min, and returned to 10% for a 2-min re-equilibration. Flow rate was 0.4 ml min−1. Pharmacokinetic parameters were calculated using noncompartmental methods in WinNonlin v.3.2 (Pharsight).
Human studies
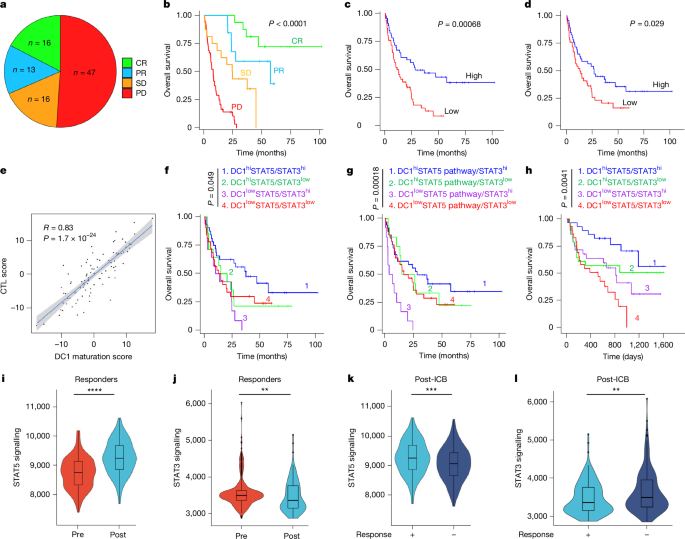
All clinical records used in this study were acquired and used with the approval of Institutional Review Boards. Patients (cohort 1) who underwent ICB therapy were recruited from the University of Michigan Hospital, Ann Arbor, MI, USA. Inclusion in the analysis applied to those who were enroled in the Michigan Center for Translational Pathology’s (MCTP) continuous comprehensive clinical sequencing programme39,47,48,49, MI-Oncoseq, and who possessed sequenced libraries of pre-treatment tumours. From the initiation of therapy, overall survival times were measured. Treatment responses were determined according to RECIST1.1 criteria50 and imRECIST51. Integrative clinical sequencing was conducted using standard protocols approved for use in MCTP’s Clinical Laboratory Improvement Amendments-compliant sequencing laboratory, as previously described47,48,52. After purification with the AllPrep DNA/RNA/miRNA kit (Qiagen), total RNA was sequenced using the exome-capture transcriptome platform on an Illumina HiSeq 2000 or HiSeq 2500 in paired-end mode. Quality control, alignment, and expression quantification was executed through the standard clinical RNA-seq pipeline, CRISP53. Read count tables were then normalized into fragments per kilobase million (FPKM) then transcripts per million (TPM) using the edgeR 4.2.2 package54. Gene scores in RNA-seq data were generated using the rank-based inverse normal transformation (INT) as demonstrated previously55. The CTL score and DC1 maturation score were generated using the following respective gene sets: (CD8A, CXCL10, CXCL9, GZMA, GZMB, PRF1, IFNG and TBX21) and (CD40, CD80, CD86, HLA-DQA1, XCR1, CLEC9A, IL12A, IL12B, HLA-DRA and IDO1). STAT5/STAT3 expression was calculated as the ratio of combined TPM of STAT5A and STAT5B (STAT5AB) to STAT3 TPM for all patients (Extended Data Table 1). Stratification was performed at the median STAT5AB/STAT3 TPM ratio and at the median DC1 maturation score. This was performed for cohort 2 (Extended Data Table 2) in each dataset, before combining group-wise. STAT5 pathway INT score was calculated using the GM-CSF reactome gene set (R-HSA-512988). STAT5 pathway/STAT3 was calculated by dividing the INT score by the STAT3 TPM in each respective sample, this value was stratified by the median.
The single-cell RNA-seq analysis of TNBC (cohort 3) (dataset GSE169246) was conducted in R using Seurat (v.4.3.0), ssGSEA, and standard data wrangling and plotting packages. All analyses were carried out after subsetting the data for immunotherapy treatment. DCs were extracted based on the original authors’ annotations. Only cells expressing 200 or more genes in at least 3 cells were included in the analysis, and cells with mitochondrial reads greater than 10% were excluded. The STAT5 signalling score for each cell was calculated by applying the GM-CSF reactome gene set (R-HSA-512988) as input to the enrichIt function from the escape (v.2.0.0) package, which implements ssGSEA. Similarly, the STAT3 signalling score was determined using the same approach. The genes used for the IL-10-driven STAT3 signalling score were obtained from previously reported gene orthologues in murine DCs56. This gene set comprised: RAP1GAP, CAMKK1, CASR, KIF1A, HPCAL4, DRAM1, RAMP2, MT2A, IGFBP6, GATA3, CXCR5, GDA, FAM65C (also known as RIPOR3), PLET1, SOCS3, MUC1 and GBP5. The DC maturation score was computed using the same method with genes canonically associated with DC maturation, including CD40, CD80, CD83, CD86, LAMP3, CCL19, IL12A, IL12B, CCL5, CCL22, CXCL9, CXCL10, NFKB1, NFKB2, NFKBIA, NFKBIB, FSCN1 and CCR7. The binary division of high and low STAT3 signalling scores was determined by visually identifying two distinct clusters. The monocyte-DCs versus conventional DC distinction was determined through a combination of iterative clustering and expression of CD14. The Wilcoxon signed-rank test was used to compare the ssGSEA scores using the stat_compare_means function from the ggpubr (v.0.6.0) package.
For the in vitro human ICB study, lin−CD45+CD11c+MHCII+ DCs were enriched and sorted from fresh cancer tissues of patients with high grade serous ovarian carcinoma. DCs (106 per ml) were pretreated with SD-36 (1 μM), washed, and co-cultured with normal peripheral blood T cells (2 × 106 per ml) in the presence of anti-human PD-L1 (10 μg ml−1), anti-CD3 (2 μg ml−1), and anti-CD28 (1 μg ml−1) for 3 days. T cell cytokine profile was analysed by FACS. For detecting the effect of SD-2301 on STAT members in human immune cells in vitro, normal human peripheral blood mononuclear cells (PBMCs) were treated with SD-2301 for 14 h, followed by immunoblotting to analyse STAT protein levels. Normal human immune cells were collected from commercial buffy coats (Carter BloodCare).
Bulk RNA-seq analysis in mouse DCs
Data processing
cDC1s were purified from BMDCs as described above. The RNA was isolated by column purification (Qiagen, 217048) with DNase treatment. The RNA-seq was conducted by BGI Genomics. Bulk RNA-seq analysis was performed using the edgeR (v.4.2.2) and limma (v.3.60.6) package workflows. Preliminary quality control measures were performed including the filtering of lowly expressed genes, and the calculation and assessment of library sizes across samples. The raw counts were then transformed to log counts per million (CPM) values.
Differential expression analysis
A design matrix was created containing cell population information (Stat3−/− versus Stat3+/+) and then contrasts for pairwise comparisons between Stat3−/− and Stat3+/+ were determined in limma using the makeContrasts function. As an additional normalization measure, removal of heteroscedasticity from the normalized counts data was achieved using the voom function. To identify differentially expressed genes, the mean-variance relationship of the normalized counts was assessed and then the normalized data were modelled as a linear combination of factors and covariates. The cutoff for the P value was P = 0.05. Differential expression analysis was performed on non-treated Stat3−/− versus Stat3+/+ cDC1s and LPS-treated Stat3−/− versus Stat3+/+ cDC1s, respectively.
Gene signature analysis
To assess the DC maturation signature, the log fold change and P values of DC maturation genes reported in the literature including (Cd40, Cd80, Cd83, Cd86, Ido1, Irf7, Tnfsf8, H2-Aa, H2-ab1, Tlr4, Il12b, Il12rb1, Il12rb2 and Cxcl9) was observed from the differential expression analysis results. To assess the significantly upregulated DC maturation pathways, gene set enrichment analysis (GSEA) was performed on the Stat3−/− versus Stat3+/+ differentially expressed genes in R using the gseGO function. To assess the significantly upregulated DC activation pathways in the DCs treated with LPS, GSEA was performed on the LPS-treated Stat3−/− versus Stat3+/+ differentially expressed genes in R using the gseGO function.
Data visualization
The z-score of the normalized counts were computed and plotted as a heat map using GraphPad Prism 10.2.2. DC maturation genes were selected from the differential expression analysis results and the log fold change and P values were plotted using GraphPad Prism 10.2.2. The GSEA plots were generated in R using the gseaplot function. The GSEA dot plot was generated by plotting key pathways from the GSEA results using the dotplot function from clusterProfiler 4.9.2 package. The volcano plot was generated by first performing differential expression analysis of LPS-treated Stat3−/− versus Stat3+/+ cDC1s and plotting log fold changes and P values of genes from the STAT5 pathway curated in the Hallmark pathway Molecular Signature Database (MSigDB).
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed using the SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling Technology, 9003s). Sheared chromatin was then immunoprecipitated with STAT5 (Cell Signaling Technology, 94205s) and IgG (Cell Signaling Technology, 2729) antibodies. SYBR green master mix (Applied Biosystems) was used to measure amplification of DNA using QuantStudio 3 Fast Real-Time PCR system (Applied Biosystems). The promoters of gene were quantified using the specific primers (Extended Data Table 3). After normalization to the Input DNA, the amount of output DNA of each target protein was calculated by subtracting that of the IgG control.
Flow cytometry analysis
Single-cell suspensions were prepared from fresh mouse tumour tissues or spleen, and lymphocytes were enriched by density gradient centrifugation. To assess intracellular cytokine production, cells were cultured for 4 h in the presence of phorbol myristate acetate (5 ng ml−1; Sigma-Aldrich), ionomycin (500 ng ml−1; Sigma-Aldrich) and brefeldin A (1: 1000, BD Biosciences). Cells were fixed and permeabilized with the Transcription factor staining buffer set (Invitrogen, 00-5523-00). Cellular phenotypes were assessed multi-parameter flow cytometry panels. Data were acquired on a BD LSRFortessa. Antibodies against the following mouse antigens were used: CD45 (30-F11, HI30), CD90 (53-2.1, 30-H12), CD3 (17A2), CD45R/B220 (RA3-6B2), CD4 (RM4-5, GK1.5), CD8 (53−6.7), IFNγ (XMG1.2), granzyme B (GB11), IL-2 (JES6-5H4), Ki-67 (B56), I-A/I-E (M5/114.15.2), CD80 (16-10A1), CD86 (GL1), H-2Kb (AF6-88.5.5.3), H-2 (M1/42), XCR1 (ZET), TNF (MP6-XT22), CD11c (HL3), phospho-Tyr694-STAT5 (SRBCZX), phospho-Tyr705-STAT3 (13A3-1). Additionally, antibodies against the following human antigens were used: CD8 (RPA-T8), IFNγ (B27) and TNF (MAb11). The strategies for DC gating and in vitro cultures and tissues are shown in Extended Data Figs. 2c and 3e, respectively. The strategy for T cell gating is presented in Extended Data Fig. 3k.
Statistics and reproducibility
Sample sizes were determined based on previously published studies to ensure appropriate statistical power. In vitro experiments were performed in at least two independent replicates. For in vivo studies, each group consisted of a minimum of five mice, which was considered sufficient to detect meaningful biological differences with good reproducibility, and mice were randomized into treatment groups at the start of the experiment. Experiments were not performed in a blinded manner, as knowledge of the treatment groups were necessary to carry out the study. t-tests were used to assess differences between two independent experimental groups, and one-way analysis of variance (ANOVA) was used to compare three or more groups. Two-way ANOVA was applied to compare tumour growth curves. Data are presented as mean ± s.e.m., and statistical significance was defined as P < 0.05 for all tests. All statistical analyses related to these experiments were performed using GraphPad Prism software v.10.2.2.
Kaplan–Meier analysis was conducted to estimate overall survival, with differences between groups assessed using log-rank tests. Cox proportional hazards models were used for multivariate survival analysis. Pearson’s correlation coefficient was applied to evaluate linear correlations between variables, and the Wilcoxon rank-sum test was performed to assess group differences. All P values are two-sided and not adjusted for multiple comparisons. The statistical analyses were conducted using R packages.
Ethics oversight
All human studies were conducted under the oversight and approval of the Institutional Review Board at the University of Michigan Medical School.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.