Protein expression and purification
POLγA (P54098), wild-type and mutant variants (R232H, A467T, W748S+E1143G, and G848S), POLγB (AAD56640.1) and the Twinkle DNA helicase (Q96RR1) were cloned and expressed as 6×His-tagged fusion proteins in Spodoptera frugiperda cells, following previously published protocols24. The SSBP1 gene encoding the human mtSSB (Q04837) was cloned into the pET-17b vector in frame with a C-terminal 6× His-tag. The mtSSB protein was subsequently purified as previously reported25.
DNA synthesis on ssDNA template
A 20-mer oligonucleotide 32P-labelled at the 5′ end (5′-GTAAAACGACGGCCAGTGCC-3′) was hybridized to M13mp18 ssDNA (New England Biolabs). Reactions were performed in 20 μl volumes, each containing 0.5 nM of template DNA, 25 mM Tris-HCl pH 8.0, 1 mM Tris(2-carboxyethyl)phosphine (TCEP), 10 mM MgCl2, 0.1 mg ml−1 bovine serum albumin (BSA), 35 mM NaCl, 10 μM of all four dNTPs (unless otherwise indicated in the figure), 1% DMSO, 200 nM mtSSB (calculated as a tetramer), 2.5 nM of the specified POLγA variants, 7.5 nM (5 nM was used in the time-course experiment) POLγB (concentration calculated as a dimer), and 1 μM PZL-A when indicated. The reactions were incubated at 37 °C for the specified duration and terminated by the addition of 4 μl stop buffer (90 mM EDTA pH 8.0, 6% SDS, 30% glycerol, and 0.25% bromophenol blue). Products were separated on a 0.8% agarose gel with 0.5 μg ml−1 EtBr at 40 V in 1× TBE buffer for 18 h and visualized by autoradiography.
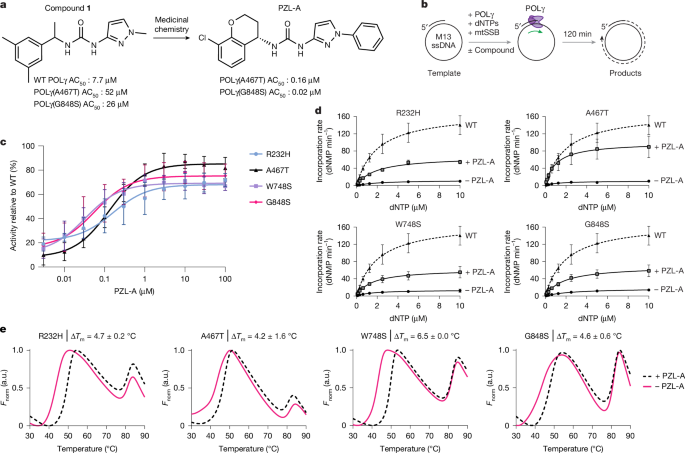
The 20-mer oligonucleotide hybridized to M13mp18 ssDNA template was also used for SYBR Green assays but without radioactive 5′-labelling of the primer. Reactions were performed at a final volume of 10 μl. Each reaction contained 0.5 nM of template DNA, 25 mM Tris-HCl pH 8.0, 1 mM TCEP, 25 mM NaCl, 10 mM MgCl2, 0.1 mg ml−1 BSA, 100 μM of all 4 dNTPs, 0.02% Triton X-100, 200 nM mtSSB (calculated as a tetramer), 0.5 nM of the specified POLγA variants, and 0.65 nM POLγB (calculated as a dimer). Duplicates of reaction mixtures were distributed into microplates (384-well) with wells containing compounds prepared from 10 mM compound stocks in 100% DMSO (final compound concentrations indicated in the figure). Equal amounts of DMSO (1% in final concentration) were added to positive and negative control reactions. The reactions were incubated at 37 °C for 2 h in a VWR INCU-Line incubator and terminated by the addition of 10 µl of 50 mM EDTA pH 8.0, 0.02% Triton X-100, and SYBR Green I (1:5,000) followed by incubation for 20 min at room temperature. The fluorescence signal was analysed using a BMG PHERAstar microtitre plate reader (between 485–520 nm) and BMG PHERAstar microtitre plate reader control software. The signal was normalized relative to wild-type (1% DMSO) and the data were plotted in Prism 10 (Graphpad Software), with errors shown as s.d., and fitted using the ‘[agonist] versus response (three parameters)’ model.
Identification of PZL-A
To identify activators that enhance POLγ activity, we screened a diverse set of about 270,000 small-molecule compounds. We adopted a homogeneous fluorescence-based method26, in which a fluorescent reporter strand is annealed to a longer, quencher-labelled template strand. DNA synthesis is initiated from a primer annealed to the same template strand and displacement of the short reporter strand is measured as an increase of fluorescence. In the initial screen we used wild-type POLγ and the threshold for hit selection was set stringently to 30% increased activity, relative to the control.
Changes in dose-response activity for wild-type POLγ and mutant derivatives during further compound optimization were assessed using the SYBR Green fluorescence assay described above. A variety of follow-up assays described in this Article were used to assess target engagement, selectivity, and cellular effects.
Steady-state kinetics
To determine apparent Km_app, dNTP and kcat, we used a 20-mer oligonucleotide hybridized to an M13mp18 ssDNA template (described above) and performed time-course experiments at 8 different concentrations of dNTPs (0.08, 0.16, 0.32, 0.625, 1.25, 2.5, 5 and 10 µM) and monitored incorporation of dNTPs. Reactions were performed in 160 µl volumes containing 0.5 nM of DNA template, 25 mM Tris-HCl pH 8.0, 1 mM TCEP, 35 mM NaCl, 10 mM MgCl2, 0.1 mg ml−1 BSA, 1.3 nM POLγA, 3.75 nM POLγB (calculated as a dimer), 200 nM mtSSB (calculated as a tetramer) and 1 µM PZL-A or 1% DMSO, and the indicated concentrations of dNTPs. To follow nucleotide incorporation, dNTPs were spiked with a small amount of [α-32P]dCTP (40 µCi (≈13 pmol) was added to 100 µl of a 200 µM dNTP solution). This dNTP solution was then diluted accordingly, both for the reactions and the calibration curve. Reactions were incubated at 37 °C and 20 µl samples were taken at 0, 2.5, 5, 7.5, 10 and 15 min and stopped by the addition of 5 µl of 0.5 M EDTA pH 8.0. The products were analysed by placing 5 µl onto a Hybond N+ positively charged membrane. The membrane was air-dried for 15 min and then washed 3 × 5 min in 2× saline-sodium citrate (SSC) buffer followed by a 2 min wash in 95% ethanol to remove non-incorporated nucleotides, air-dried again and visualized by autoradiography and quantified using the Fujifilm Multi Gauge V3.1 software. A standard curve was generated by adding 5 µl of each indicated amount of dNTP onto a membrane, which was dried without washing representing the total amount of dNTPs.
To determine the apparent Kd for interactions between PZL-A and POLγ (Kd_app, PZL-A), we performed time-course experiments (0, 2.5, 5, 7.5, 10 and 15 min) at different concentrations of PZL-A (1.37, 4.1, 12.3, 37, 111, 333 and 1,000 nM) with a fixed concentration of 20 µM dNTP (including 0.4 µCi (≈133 fmol) [α-32P]dCTP) (Table 1). The experiments were performed as described for the steady-state kinetics experiments.
Initial rates were determined for the different dNTP and PZL-A concentrations used. For the dNTP titration experiments, the rates were normalized by dividing by the concentration of active enzyme–DNA complex (see ‘Determination of active POLγ–DNA complex’). Velocity was plotted (using Prism 10) against [dNTP] or [PZL-A], and non-linear regressions were performed using either the ‘Michaelis–Menten’ or ‘one site—specific binding’ models to obtain values for Km_app, dNTP and kcat or Kd_app, PZL-A. All reactions were performed at least three times.
Determination of active POLγ–DNA complex
The concentration of active enzyme–DNA complex was determined by performing EMSA as described in ‘The DNA-binding activity’ but using the same conditions as in the kinetics assays. This was accomplished by using 0.5 nM template DNA in presence of 1.3 nM POLγA and increasing amounts of POLγB (0, 5, 10, 20, 40, 60, 100 and 200 fmol POLγB in 15 μl reactions). While POLγA alone binds the DNA and generates a shift, the addition of POLγB generates a slower migrating shift which represents the active complex. As the amount of POLγB increases, the amount of active protein also increases until it reaches a plateau (saturated POLγA). At the POLγB:POLγA ratio 3:1 (POLγB calculated as dimer) the system reached the conditions used in the kinetics assays. By quantification of bound and unbound DNA we could determine the concentration of active protein–DNA complex (Extended Data Fig. 1b). These values were used to normalize the rates in the steady-state kinetics assay (dNTP titration) and to calculate the Vmax as presented in Table 1.
Exonuclease activity
To measure the 3′-to-5′ exonuclease activity of POLγA, a 20-mer oligonucleotide (5′-GCGGTCGAGTCCGGCGGCGC-3′) was 32P-labelled at the 5′ end and annealed to a 36-mer oligonucleotide (5′-GACTACGTCTATCCGGACGCCGCCGGACTCGACCGC-3′), resulting in a 19-bp dsDNA region with a single-nucleotide mismatch at the 3′ end. Reaction mixtures (10 μl) contained 25 mM Tris-HCl pH 8.0, 1 mM DTT, 10 mM MgCl2, 0.1 mg ml−1 BSA, 8.7% glycerol, 1 nM template, 2 nM POLγA, 4 nM POLγB (calculated as a dimer), 1% DMSO, and when indicated, 1 μM PZL-A. Reactions were incubated at 37 °C for the indicated duration and terminated by the addition of 10 μl stop buffer (98% formamide, 10 mM EDTA pH 8.0, 0.025% bromophenol blue, and 0.025% xylene cyanol). The products were separated by electrophoresis in 7 M urea/10% polyacrylamide gels for 2 h at 1500 V and visualized by autoradiography. The time required for the exonuclease activity to degrade the mismatch nucleotide (C), and the subsequent nucleotide (G), was quantified using the Fujifilm Multi Gauge V3.1 software. s.d. values were calculated from triplicate experiments to represent variability.
Differential scanning fluorimetry
The fluorescent dye SYPRO Orange was used to monitor temperature-induced unfolding of POLγ as previously described27. In brief, the experiment was performed in 384-well PCR plates and individual reactions contained wild-type or mutant proteins (final concentration 0.5 μM), 5× SYPRO Orange, 20 mM Tris-HCl pH 8.0, 100 mM NaCl and 1 mM DTT, in the absence or presence of 10 μM PZL-A. Differential scanning fluorimetry was performed in a CFX Opus 384 Real-Time PCR System using the CFX Maestro real-time software (Bio-Rad). Scans were recorded using the HEX emission filter (560–580 nm) between 4 and 95 °C in 0.5 °C increments with a 5 s equilibration time. The melting temperature (Tm) was determined from the first derivative of a plot of fluorescence intensity versus temperature. ΔTm was determined by subtracting Tm (without PZL-A) from Tm (with PZL-A). The s.d. of ΔTm was calculated from three independent measurements.
Cryo-electron microscopy sample preparation and data acquisition
Primer template DNA was formed by annealing a 25-nt primer (5′-GCATGCGGTCGAGTCTAGAGGAGCC-3′) to a 40-nt template strand (5′-TTTTTTTTTTATCCGGGCTCCTCTAGACTCGACCGCATGC-3′). To prepare cryo-EM samples, POLγA (A467T, G848S, or wild-type) was mixed at a 1:2 molar ratio with POLγB and dialysed into a buffer containing 20 mM HEPES-NaOH pH 7.5, 140 mM KCl, 10 mM CaCl2 and 1 mM TCEP. Following dialysis, PZL-A was added to a final concentration of 20 μM and incubated for 10 min at room temperature (this step was omitted for the apo samples). Primer template DNA was added at a POLγ:DNA molar ratio of 1:1.2, and dCTP was added to a final concentration of 0.2 mM. The samples were incubated on ice for 10 min before being applied to the grids. Grids were prepared using a Vitrobot Mark IV (Thermo Fisher Scientific) by adding 3.5 μl of approximately 2 μM protein sample to glow-discharged QuantiFoil 2/1 grids (Quantifoil Micro Tools GmbH) at 4 °C and 100% humidity. Grids were blotted for 2 s and plunge-frozen in liquid ethane.
Cryo-EM data were collected on two Titan Krios G2 microscopes (Thermo Fisher Scientific) at the SciLifeLab cryo-EM facility in Stockholm, both operated at 300 kV and equipped with a K3 direct electron detector (Gatan) and a BioQuantum energy filter (Gatan) set to a slit width of 20 eV. Five cryo-EM datasets (G848S-PZL-A, G848S, A467T-PZL-A, A467T, and wild-type) were collected in super-resolution mode at a nominal magnification of 105,000×, corresponding to a calibrated pixel size of 0.825 Å or 0.828 Å. The total dose was 40–42 e− Å−2 per 40 frames, and the movies were acquired at a defocus range of −0.8 μm to −2.2 μm.
Cryo-electron microscopy data processing
The processing workflows for G848S-PZL-A, G848S, A467T-PZL-A, A467T, and wild-type POLγ are shown in Extended Data Figs. 2–4. For the five datasets, the acquired movie stacks were imported into cryoSPARC (v4.3.1) for image processing28. Motion correction of the data was performed with Patch Motion Correction, and the contrast transfer function (CTF) was estimated with Patch CTF Estimation. Micrographs with poor CTF fit (worse than 4 Å) were removed. The Automatic Blob Picker was used to pick particles, which were extracted with 4× binning (3.3 Å per pixel). Multiple rounds of two-dimensional (2D) classifications were performed to filter out junk particles. The remaining particles were used to perform a three-class ab initio reconstruction followed by heterogeneous refinement. At this stage, for all five datasets, there was at least one class with clear POLγ features. These classes were kept and filtered further with several rounds of 2D classifications and heterogeneous refinements with one junk volume to remove poor particles. The particles and volume from the POLγ class for each of G848S-PZL-A, A467T-PZL-A, and A467T were refined with non-uniform refinements and local/global CTF refinements, resulting in final 3D reconstructions with a gold-standard Fourier shell correlation (GSFSC) resolution of 2.63 Å, 2.67 Å and 2.69 Å, respectively. During the processing of G848S and wild-type POLγ, two POLγ classes emerged after heterogeneous refinement. However, in both cases, one of these classes was anisotropic due to accumulation of side views and not used for the final reconstructions. The other class, for both G848S and wild-type, was refined with non-uniform refinements and local/global CTF refinements, resulting in final 3D reconstructions with a GSFSC resolution of 2.39 Å and 2.65 Å, respectively. The GSFSC = 0.143 criterion was used for determining the resolution of all reconstructions. All final maps were sharpened using DeepEMhancer and Phenix Autosharpen29,30.
Model building, refinement and analysis
To build the wild-type and mutant POLγ structures, the POLγ ternary complex (PDB ID: 4ZTZ) was docked into the cryo-EM maps by rigid body fitting in UCSF ChimeraX (v.1.4) and manually fitted in real space in Coot (v.0.9.8.1) and ISOLDE (v.1.4)31,32,33. In the mutant models (A467T and G848S), the amino acids were mutated in Coot. In the cryo-EM maps generated from samples with PZL-A added, an unaccounted density was identified between the POLγA and the proximal POLγB unit, where PZL-A could be fitted. Coordinates and restraints for PZL-A were created using the eLBOW module in PHENIX (v.1.20) and positioned in the density34,35. After initial fitting, the models were improved iteratively with real-space refinement in PHENIX and manual adjustments in Coot. The refined models were validated with MolProbity (Extended Data Table 1)36. Local resolution was estimated for all five maps with cryoSPARC (Fourier shell correlation (FSC) threshold = 0.5) (Extended Data Figs. 2–4). Figures were prepared using UCSF ChimeraX.
The DNA-binding activity
DNA binding of POLγ holoenzyme (POLγA and POLγB in complex) to a primer template was assayed using a 36-mer oligonucleotide (5′-TTTTTTTTTTATCCGGGCTCCTCTAGACTCGACCGC-3′) annealed to a 5′-32P-labelled 21-mer complementary oligonucleotide (5′-GCGGTCGAGTCTAGAGGAGCC-3′). This produces a primed template with a 15-base single-stranded 5′ tail. Reactions were carried out in 15 μl volumes containing 0.5 nM DNA template, 25 mM Tris-HCl pH 8.0, approximately 30 mM NaCl, 1 mM TCEP, 0.1 mg ml−1 BSA, 10% glycerol, 10 µM ddCTP and either 1% DMSO or 1 µM PZL-A. POLγB (6.6 nM, calculated as a dimer) was included in the mixture and POLγA (0, 0.011, 0.021, 0.042, 0.083, 0.17, 0.33, 0.67 and 1.33 nM) was added as indicated in the figures. Reactions were incubated on ice for 10 min followed by 10 min at room temperature before separation on a 6% native PAGE gel in 0.5× TBE for 40 min at 180 V. Bands were visualized by autoradiography.
For Kd analysis, band intensities representing unbound and bound DNA were quantified using Multi Gauge V3.0 software (Fujifilm Life Sciences). The fraction of bound DNA was calculated from the background-subtracted signal intensities using the expression: bound/(bound + unbound). The fraction of DNA bound in each reaction was plotted against the concentration of POLγ. Data were fitted using the quadratic equation (fraction DNA bound = (([POLγtot] + [DNAtot] + Kd) – sqrt(([POLγtot] + [DNAtot] + Kd)2 − 4[POLγtot] × [DNAtot]))/2[DNAtot]) in Prism 10 (Graphpad Software) to obtain values for Kd. Each Kd value is presented as the average of three independent reactions.
For the off-rate measurements, the same primer template used in the cryo-EM studies was used but the 25-nucleotide primer was 32P-labelled in the 5′ end. The reactions contained 5 nM of indicated POLγA variants, 7.5 nM POLγB, 0.6 nM 32P-labelled primed template, 25 mM Tris-HCl pH 7.8, 10 mM MgCl2, 0.1 mg ml−1 BSA, 10 mM DTT, 8.5% glycerol, 100 μM dGTP, and 100 μM dCTP or ddCTP in a final volume of 15 μl. When indicated, 1 µM PZL-A was added. The mixture was preincubated at room temperature for 10 min, followed by the addition of a 50× excess cold (non-labelled) primer template, and incubated at 37 °C for indicated time. The samples were separated on a 4% native polyacrylamide gel (0.5× TBE) for 20 min at 180 V and visualized by autoradiography. Band intensities representing unbound and bound DNA were quantified using Multi Gauge V3.0 software (Fujifilm Life Sciences). The remaining fraction of bound DNA was determined from the background-subtracted signal intensities, using the expression: bound/(bound + unbound), and normalized against the value at t = 0. DNA bound (in %) in each reaction was plotted versus time (min). Data were fit using the “dissociation–one phase exponential decay” model in Prism 10 (Graphpad Software) to obtain the dissociation rate constant, koff, with errors shown as s.e.m.
Processivity measurements
The same template as in DNA synthesis on ssDNA template described above was used in a reaction mixture A (10 μl) containing 50 mM Tris-HCl pH 8.0, 2 mM TCEP, 0.2 mg ml−1 BSA, 50 mM NaCl, 2% DMSO, 5 nM template, 2.5 nM of the specified POLγA variants, 5 nM POLγB (calculated as a dimer), and, when indicated, 2 μM PZL-A. The A mixtures were incubated for 10 min on ice prior to adding mixture B (10 μl) containing 20 mM MgCl2, 20 μM of all four dNTPs, and 600 ng ml−1 heparin, and then immediately incubated at 37 °C for 5 min. Reactions were stopped by adding 20 μl stop buffer (95% formamide, 20 mM EDTA pH 8.0, and 0.1% bromophenol blue). Samples were heated at 95 °C for 3 min before loaded on a pre-run (1 h, 1,500 V) 8% denaturing PAGE-UREA (6 M Urea, 1× TBE) sequencing gel and run for 75 min at 1,500 V in 1× TBE buffer. Replication products were visualized by autoradiography.
Rolling-circle replication
A dsDNA template with a preformed replication fork was generated by annealing a 70-mer oligonucleotide (5′-T42ATCTCAGCGATCTGTCTATTTCGTTCAT-3′) to single-stranded pBluescript SK(+) OL, followed by one cycle of polymerization with KOD polymerase, as previously described24. The template (0.4 nM) was added to a reaction mixture (final volume 25 μl) containing 25 mM HEPES-NaOH, pH 7.6, 10 mM DTT, 10 mM MgCl2, BSA (0.1 mg ml−1), 1 mM ATP, 10 μM of all four dNTPs, 2 μCi of [α-32P]dCTP, 1% DMSO, 4 nM Twinkle (calculated as a hexamer), 160 nM mtSSB (calculated as a tetramer), 6 nM POLγA (wild-type or mutant variants), and 9 nM POLγB (calculated as a dimer). When indicated, 1 μM PZL-A was included in the reactions. After incubation at 37 °C for times indicated, reactions were terminated by adding 8 μl of alkaline loading buffer (18% (w/v) Ficoll, 300 mM NaOH, 60 mM EDTA pH 8.0, 0.25% (w/v) bromophenol blue, and 0.25% (w/v) xylene cyanol FF) and separated on a 0.8% denaturing agarose gel at 40 V for 20 h. The experiments were performed in triplicate. Reaction products were visualized by autoradiography and quantified using Fujifilm Multi Gauge V3.1 software. Standard deviations were calculated to represent variability across the triplicate experiments.
Cell lines
Primary skin fibroblasts from two patients with mutations in POLG (NP_001119603.1) were obtained from the Swedish Biobank. Patient 1 exhibited compound heterozygosity for mutations p.[Ala467Thr] and [Gly848Ser], and patient 2 harboured compound heterozygous mutations p.[Trp748Ser+Glu1143Gly] and p.[Arg232His]. Primary skin fibroblasts from donors with wild-type POLγ were used as controls. Genotypes were confirmed via PCR amplification followed by Sanger sequencing. Fibroblasts were cultured in Dulbecco’s Modified Eagle Medium (DMEM; 4.5 g l−1 glucose, 4 mM glutamine, 110 mg l−1 sodium pyruvate) supplemented with 10% fetal bovine serum at 37 °C in a 5% CO2 humidified cell incubator. All cell lines were authenticated by STR profiling, with no common misidentified lines detected. They were routinely tested for mycoplasma contamination using PCR, and all results were confirmed negative.
A human induced pluripotent stem (hiPS) cell line (PGP1-SV1) was reprogrammed from PGP1 fibroblasts and used as a control for all sequential stem cell-related experiments. The PGP1 hiPS cell line was derived from fibroblasts obtained from G. Church as part of the Personal Genome Project (PGP), an initiative dedicated to open-access genomic research. The fibroblast samples were collected with informed consent, explicitly permitting the public posting and unrestricted commercial use of Personally Identifying Genetic Information (PIGI). The donors signed an informed consent form that allows for open-access distribution of genetic and cellular data, including whole-genome SNP arrays, whole-exome sequences, and whole-genome sequences. The PGP1 cells have no commercial restrictions under the Open Material Transfer Agreement and are freely available for research use. The PGP1 cell line has been previously characterized and is described in the patent: Methods for increasing efficiency of nuclease-mediated gene editing in stem cells37.
The PGP1 fibroblasts were reprogrammed into hiPS cells using a non-integrating Sendai viral approach with the CytoTune-iPS 2.0 Sendai Reprogramming Kit (Thermo Fisher Scientific). This method allows for efficient reprogramming without genomic integration, ensuring the maintenance of genomic stability.
To model POLG-related mitochondrial disease, a homozygous POLG mutation p.[Gly848Ser] was introduced into the PGP1 hiPS cell line using CRISPR–Cas9 gene-editing technology (Synthego). Mutations were confirmed by PCR amplification followed by Sanger sequencing.
Cell viability assay
Relative cell viability of primary skin fibroblast cell lines derived from POLG patients was assessed using the MTT Cell Proliferation Kit I (Roche Diagnostics, 11465007001) following the manufacturer’s protocol. Cells were treated with compound concentrations ranging from 0 to 10 μM (0, 0.003, 0.01, 0.03, 0.1, 0.3, 1, 3 and 10 μM) for 120 h, with treatment starting 24 h after cell seeding. Vehicle (0.1% DMSO) served as a control.
mtDNA depletion and recovery in proliferating cells
mtDNA depletion was induced by adding 50 ng ml−1 EtBr to the culture medium for up to 7 days followed by a return to EtBr-free medium. Cell pellets were collected at time points indicated in figure legends for subsequent DNA extraction during EtBr treatment. After removal of EtBr, cells were maintained at 70–90% confluence to ensure exponential growth and split every 3–4 days with fresh medium containing PZL-A or vehicle (0.1% DMSO). The treatment duration extended up to 12 days, as indicated in the figure. PZL-A was added at concentrations of 3 μM to POLγ(A467T/G848S) or wild-type POLγ cells and 1 μM to POLγ(W748S/R232H) mutant cells, and vehicle served as a control. Cell pellets were collected at specified time points for subsequent DNA extraction. EtBr-untreated cells served as non-depleted controls in parallel experiments. No differences in cell doubling times were observed during EtBr treatment or the repopulation phase of the experiment. All cell culture studies were conducted in triplicate unless otherwise stated in the figure legends.
mtDNA depletion and recovery in quiescent cells
Fibroblast cells were seeded in 12-well plates, and mtDNA depletion was induced by adding 50 ng ml−1 EtBr to the culture medium for 7 days, followed by a return to EtBr-free medium. After 7 days of EtBr treatment, the cells reached confluence. EtBr was then withdrawn, and the FBS concentration was reduced to 0.1% to induce quiescence. Three days later, the cells were treated with either vehicle (0.1% DMSO) or 1 μM PZL-A for 7 or 14 days. During treatment, the medium containing PZL-A or vehicle was refreshed every 3–4 days. DNA was extracted at various time points, as indicated in the figure legends, and mtDNA levels were quantified by qPCR. EtBr-untreated cells served as non-depleted controls in parallel experiments. Experiments were performed in three independent biological replicates.
Neuronal stem cell culture and treatments
G848S/G848S mutant and PGP1 hiPS cells were cultured and expanded in mTeSR Plus complete medium (Stemcell Technologies, 100-0276) on iMatrix511 coated plates (ReproCell, NP892-011) and passaged using StemPro Accutase (Thermo Fisher Scientific, A1110501). hiPS cells were differentiated into neural stem cells (NSCs) under feeder-free conditions in 3% CO2 (Creative Biolabs).
G848S/G848S mutant and PGP1 NSCs were cultured on Matrigel-coated dishes in StemDiff Neural Progenitor Medium (Stemcell Technologies, 05833) supplemented with 5 μM Y27632 (Stemcell Technologies, 72304). Cells were maintained in a 37 °C humidified incubator with 5% CO2.
NSCs were treated with 0.2 µM or 1 µM PZL-A, or vehicle (0.01% DMSO), for 10 days. During the treatment period, cells were passaged every 3–4 days using Accutase and cultured in fresh medium containing PZL-A or vehicle. After 10 days of treatment, cells were detached with Accutase and replated at 30,000 cells per well onto Matrigel-coated Seahorse XFe96 Pro plates. The Agilent Mito Stress Test was performed as described in the Metabolic Flux Assay section, and cell numbers per well were determined using the Invitrogen CyQuant assay. Parallel cell pellets collected after 10 days of treatment were used for DNA extraction and mtDNA quantification. All experiments were performed in three independent biological replicates.
mtDNA quantification
Cell pellets were lysed in lysis buffer (100 mM NaCl, 25 mM EDTA pH 8.0, 10 mM Tris-HCl, pH 8.0, 0.5% SDS) and digested with Proteinase K (100 ng ml−1) at 55 °C for 1 h. Genomic DNA was extracted using Zymo Genomic DNA Clean and Concentrator purification kit according to the manufacturer’s instructions (Zymo Research, D4010). Prior to elution, DNA was treated with RNase A (100 ng ml−1). The mtDNA quantification was measured using real-time qPCR with iTaq Universal SYBR Green Supermix (Bio-Rad, 1725124). Specific primers targeting the mitochondrial gene MT-CYB (5′-ACAATTCTCCGATCCGTCCC-3′ and 5′-GTGATTGGCTTAGTGGGCGA-3′) were used for mtDNA quantification, and primers for the nuclear 18S rRNA (5′-AGAAACGGCTACCACATCCA-3′ and 5′-CCCTCCAATGGATCCTCGTT-3′) or B2M gene (5′-TGCTGTCTCCATGTTTGATGTATCT-3′ and 5′-TCTCTGCTCCCCACCTCTAAGT-3′) served as a loading control.
Southern blotting was also performed to evaluate mtDNA and 7S DNA levels following mtDNA depletion and recovery in the presence of PZL-A during the proliferation phase. Genomic DNA (2 μg) was digested at 37 °C overnight using 20 U of the BamHI-HF restriction enzyme. Digested DNA was separated by electrophoresis on a 1% agarose gel, followed by depurination in 0.25 M HCl for 20 min. The DNA was denatured in 0.5 M NaOH and 1.5 M NaCl, neutralized in 0.5 M Tris-HCl, pH 7.4, and 1.5 M NaCl, and transferred overnight onto a Hybond N+ nitrocellulose membrane. Cross-linking was performed using 254-nm ultraviolet light at 200 mJ cm−². The membrane was sequentially hybridized with probes detecting mtDNA and nuclear 18S DNA. Radiolabelled dsDNA probes were prepared by random primer labelling (Agilent, 300385) of gel-extracted human mtDNA (5′-CTCACCCACTAGGATACCAAC-3′ and 5′-GATACTGCGACATAGGGTGC-3′) and 18S rDNA (5′-AGAAACGGCTACCACATCCA-3′ and 5′-CCCTCCAATGGATCCTCGTT-3′) PCR products. After hybridization, radiolabelled signals were detected using a FLA-7000 phosphorimager (version 1.12) and analysed with Multi Gauge software (version 3.0, Fujifilm).
In organello replication
Freshly isolated mitochondria (1 mg) were resuspended in 1 ml of incubation buffer (25 mM sucrose, 75 mM sorbitol, 100 mM KCl, 10 mM K2HPO4, 0.05 mM EDTA pH 8.0, 5 mM MgCl2, 1 mM ADP, 10 mM potassium glutamate, 2.5 mM potassium malate, 10 mM Tris-HCl, pH 7.4) supplemented with 1 mg ml−1 fatty acid-free BSA. The mixture was supplemented with 50 μM each of dTTP, dATP, and dGTP along with 20 μCi of [α-32P]dCTP (3,000 Ci mmol−1) and incubated at 37 °C for 2 h on a rotating wheel. Following incubation, mitochondria were pelleted at 7,600g for 4 min and washed twice with washing buffer (10% glycerol, 10 mM Tris-HCl, pH 6.8, 0.15 mM MgCl2). mtDNA was extracted using phenol followed by ethanol precipitation.
Resuspended mtDNA samples were heated to 95 °C for 2 min to release 7S DNA from mtDNA and then separated on a 1% agarose gel. The dried agarose gel was exposed to a phosphorimager screen for visualization. For loading controls, protein extracts from equal aliquots of mitochondria were prepared and subjected to VDAC immunoblotting.
Immunoblotting
Cell pellets were incubated in a cell lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA pH 8.0. 1% Triton X-100, supplemented with 1× protease inhibitors: 2 mM benzamidine, 2 μM pepstatin A, 1 mM phenylmethylsulfonylfluoride, 0.5 μM leupeptin) on ice for 30 min. The lysate was then centrifuged at 11,000g for 10 min 4 °C, and the supernatant containing soluble proteins was collected. Protein samples were then separated by SDS–PAGE using gradient gels (4–20%) and transferred onto nitrocellulose membranes for immunoblotting. Membranes were probed with total OXPHOS antibody cocktail (abcam, ab110413), VDAC (abcam, ab14734), POLγA (abcam, ab128899) or tubulin (Sigma, T5168) as figure legend indicated.
Metabolic flux assay
The experimental method followed the Agilent Seahorse metabolic flux analyser guidelines. In brief, patient fibroblasts depleted of mtDNA and treated with 1 μM PZL-A or vehicle for 7 days were prepared as described previously.
One day prior to the experiment, an XFe96 Pro cartridge was hydrated with 200 μl of calibrant solution per well and left overnight at 37 °C. On the day of the experiment, cells were seeded at 2 × 104 cells per well in DMEM growth medium in a pre-coated Seahorse XFe96 Pro Cell Culture Microplate. The plate was incubated at 37 °C in a CO2 incubator for 2 h to allow cell adhesion.
The growth medium was then replaced with Seahorse Phenol Red-free DMEM supplemented with 5 mM glucose, 2 mM glutamine, and 1 mM sodium pyruvate. Cells were incubated for 1 h at 37 °C to equilibrate in the Seahorse medium. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured using the XF Cell Mito stress test kit (Agilent, 103015-100) following the manufacturer’s instructions. Specifically, mitochondrial inhibitors (1 μM oligomycin A, 1.5 μM rotenone/antimycin A) or an uncoupler (2 μM FCCP) were diluted in Seahorse medium and loaded into the appropriate ports on the cartridge just before calibration of the Seahorse analyser and subsequent analysis of the cell plate.
Following completion of the Seahorse assay, all medium was removed from the plate and the plate was frozen at −80 °C overnight. For cell quantification, this plate was thawed, and the CyQuant assay (Thermo Fisher, C7026) was performed. Lysis buffer was diluted 1:20 in water and supplemented with CyQuant dye (1:400 dilution). 200 μl of this mixture was added to each well and incubated for 5 min protected from light. Fluorescence was measured using a BMG Labtech Spectrostar plate reader, and cell numbers were quantified using a standard curve. Data analysis and normalization were conducted using Agilent Seahorse Wave Pro software and Prism 10.
Statistical analysis
All numerical data are expressed as mean ± s.d. or s.e.m. as stated in the figure legends. Unpaired two-tailed Welch’s t-test was used to assess statistical significance for comparisons between two groups. For multiple comparisons, Welch and Brown–Forsythe one-way ANOVA was used. Differences were considered statistically significant at P < 0.05.
Ethics statement
The study was conducted according to the Declaration of Helsinki of 1975.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.