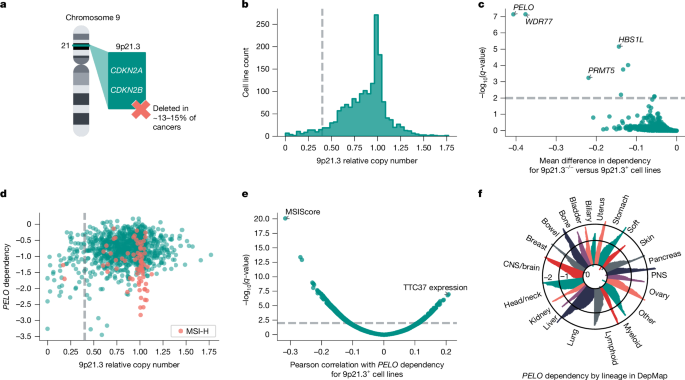
Homozygous deletion of chromosomal region 9p21.3 is among the most frequently observed somatic copy number alterations in human cancers, occurring in approximately 13–15% of all cancers4,5 (Fig. 1a). Many 9p21.3-loss cancers are associated with poor clinical outcomes, including subsets of glioblastoma, urothelial carcinoma of the bladder, pancreatic ductal adenocarcinoma, oesophageal adenocarcinoma and non-small-cell lung cancer6,7,8,9,10,11. Loss of tumour suppressors CDKN2A or CDKN2AB is thought to be a key driver of 9p21.3 deletions (9p21.3−/−) in cancers. However, recent studies suggest that deletions of neighbouring genes are also important for oncogenesis, which might explain why this chromosomal region is deleted rather than isolated inactivation of CDKN2A or CDKN2B MTAP, which lies adjacent to CDKN2A, is a putative tumour suppressor gene that is frequently codeleted with CDKN2A and CDKN2B12,13. Studies have also linked the loss of an interferon gene cluster on 9p21.3 to immune evasion and primary resistance to immune checkpoint inhibitors5,14.
a, Illustration of chromosome 9 cytoband 9p21.3, which is biallelically deleted (9p21.3−/−) in approximately 13–15% of human cancers. b, Histogram of 9p21.3 relative copy number across DepMap cell lines (n = 1,750). Threshold of less than 0.4 (dashed line) indicates cell lines characterized as 9p21.3−/−. c, q-values from left-tailed Student’s t-test between 9p21.3−/− (less than 0.4 threshold, n = 63 cell lines) or at least one intact copy of 9p21.3 (9p21.3+, n = 1,037 cell lines) plotted against the mean difference in gene dependency in DepMap. d, PELO dependency plotted against 9p21.3 relative copy number (n = 1,100 cell lines), with MSI-H cell lines (MSIsensor2 MSIScore > 20, n = 73) highlighted. e, Univariate associations of DepMap omics data with PELO dependency score in 9p21.3+ cell lines (n = 1,037). f, PELO dependency score for cell lines (n = 1,100) grouped by OncoTree lineage. Ovary, ovary and/or fallopian tube; bladder: bladder and/or urinary tract; soft, soft tissue; stomach, oesophagus and/or stomach; head/neck, head and neck; PNS, peripheral nervous system; biliary, biliary tract; ampulla, ampulla of Vater. Relative copy number, gene dependency and omics data are from DepMap 23Q4 or 24Q2 release.
Large deletions of chromosomal regions can provide opportunities for cancer treatment. In particular, codeletion of passenger genes in proximity to tumour suppressors can lead to collateral lethality and create potential vulnerabilities specific to those cancers15,16,17,18. These codeleted genes may have critical roles in cell maintenance, and their loss can lead to newly acquired dependencies on related or paralogous genes. For instance, large-scale RNA interference data from the Cancer Dependency Map (DepMap) and Project Drive led to the discovery of PRMT5 as a promising target in 9p21.3−/− cancers through its synthetic lethal interactions with MTAP deletion19,20. Although clinical trials of PRMT5 inhibitors and related MAT2A inhibitors for patients for MTAP-deleted cancers are underway, the frequency, poor outcomes and immune checkpoint inhibitor resistance associated with 9p21.3−/− cancers highlight an urgent need for further therapeutic strategies for this broad class of cancers21,22.
Here, we used large-scale CRISPR knockout screening datasets from DepMap to identify further synthetic lethal targets associated with 9p21.3−/− in cancer23. Using data from the DepMap 23Q4 release, we first computed the relative copy number for 9p21.3 for each cell line using a weighted average across the cytoband24 (Fig. 1b). We tested several thresholds for calling 9p21.3 loss and performed left-tailed Student’s t-tests comparing CRISPR gene effect scores for cell lines above or below each 9p21.3 relative copy number threshold (Fig. 1b and Extended Data Fig. 1a). Each analysis using a 9p21.3 relative copy number threshold of 0.5 or less identified PELO as the top preferential dependency in cell lines with low relative 9p21.3 copy number (effect size = −0.406, n = 1,100, q = 7.58 × 10−8; Fig. 1c and Extended Data Fig. 1a). We also observed that cell lines with greater loss of 9p21.3 were more likely to be preferentially dependent on PELO for viability. For the remainder of our analyses, we used a relative copy number threshold of 0.4 to distinguish models with 9p21.3−/− (less than 0.4, n = 63) versus models with at least one intact copy of 9p21.3 (9p21.3+, greater than or equal to 0.4, n = 1,037) (Fig. 1b). Our analysis also identified HBS1L as a preferential vulnerability in 9p21.3−/− cell lines (left-tailed Student’s t-test, effect size = −0.144, n = 1,100, q = 7.10 × 10−6). As PELO interacts with HBS1L to recruit ACBE1 to promote dissociation of stalled ribosomes and release intact peptidyl-transfer RNA, this observation supported our hypothesis that PELO is preferentially required in 9p21.3−/− cancers25,26 (Fig. 1c and Extended Data Fig. 1a). As expected, our analyses also recovered the PRMT5–WDR77 complex, a previously described synthetic lethal dependency with loss of the 9p21.3 gene MTAP, for nearly all thresholds tested19,27 (left-tailed Student’s t-test for threshold of 0.4; PRMT5: effect size = −0.220, n = 1,100, q = 5.76 × 10−4; WDR77: effect size = −0.377, n = 1,100, q = 7.54 × 10−8; Fig. 1c and Extended Data Fig. 1a).
We observed that some 9p21.3+ cell lines were dependent on PELO for survival (Fig. 1d). To determine whether there were any other genomic features associated with PELO dependency, we analysed DepMap expression, copy number, mutation and genomic signature data for correlation with PELO dependency. According to this analysis, the MSIsensor2 MSIScore, a measure of MSI from next-generation DNA sequencing, was the top correlated feature associated with PELO dependency in 9p21.3+ cells28 (Pearson’s R = −0.316, n = 1037, q = 9.52 × 10−21; Fig. 1e). MSI-H (MSIsensor2 score > 20) is a hypermutable state observed in substantial subsets of colon, endometrial, gastric and ovarian cancers9,29,30. We next compared 9p21.3+/MSI-H and 9p21.3+/microsatellite stable (MSS) cell lines and found that PELO scored as a strong preferential dependency in MSI-H cell lines, second only to the previously described synthetic lethal target Werner helicase31,32 (two-tailed Student’s t-test; PELO: effect size = −0.500, n = 1037, q = 1.45 × 10−16; Werner helicase: effect size = −0.691, n = 1037, q = 2.02 × 10−72; Extended Data Fig. 1b). PELO dependency was highly variable across many cancer lineages, with those associated with MSI-H or 9p21.3−/− enriched for strong PELO dependency (Fig. 1f). Collectively, these data raise the hypothesis that cancers with one of two predictive biomarkers, biallelic large 9p21.3 deletions or MSI-H, preferentially require PELO for survival.
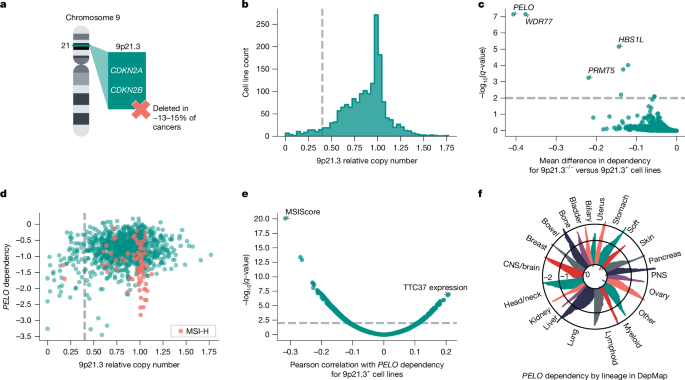
As a first step of validation, we interrogated the viability effects of knocking down PELO in ten different cell lines with CRISPR interference (CRISPRi). These cell lines consisted of a panel representing 9p21.3+/MSS (two cell lines), 9p21.3−/−/MSS (three cell lines) or 9p21.3+/MSI-H (three cell lines). In addition, we included SF295 and KP4, cell lines that harbour short deletions in 9p21.3, although they were scored as 9p21.3+ according to our criteria. We used dCas9–KRAB, a catalytically dead Cas9 fused to the transcriptional repressor KRAB, and three different CRISPRi guide RNAs (gRNA 1, 2 and 3) to knock down PELO33. PELO knockdown strongly impaired the viability of 9p21.3−/−/MSS (left-tailed Student’s t-test, effect size = −1.20, n = 85, P = 8.52 × 10−20) and 9p21.3+/MSI-H (left-tailed Student’s t-test, effect size = −1.10, n = 66, P = 1.42 × 10−18; Fig. 2a and Extended Data Fig. 2a,b) cell lines, approximating the effects of pan-essential control gRNAs targeting SF3B1 or POLR2D. By contrast, for 9p21.3+/MSS or 9p21.3 short deletion/MSS cell lines, PELO knockdown showed no significant difference in terms of effect on viability compared with negative control gRNAs targeting an intergenic region on chromosome 2 (Ch2-2) and/or an empty vector control (left-tailed Student’s t-test, effect size = −0.03, n = 112, P = 0.299). These results provide orthogonal data that PELO is a preferential dependency in cell lines with large deletions of 9p21.3 and MSI-H cell lines.
a, Viability effect of CRISPRi-mediated PELO knockdown (KD) normalized to average of negative controls (grey dashed line; empty vector and/or gRNA Ch2-2) and positive controls (orange dotted line; gRNA for SF3B1 and/or POLR2D) for 9p21.3+ or short deletion/MSS (n = 4), 9p21.3−/−/MSS (n = 3) and 9p21.3+/MSI-H cell lines (n = 3). b, Top, immunoblots of PELO and vinculin in MIA PaCa-2 cells transduced with the indicated gRNA ± PELO cDNA. Bottom, viability scores normalized to average viability of negative controls. c, Immunoblots of PELO and vinculin levels in the indicated patient-derived tumour organoid models. Relative viability following DOX-induced knockdown of the indicated gRNA, relative to DMSO control. d, Average tumour volume over time for nude mice with subcutaneous engraftment of MIA PaCa-2 cells following randomization to a standard diet (control) or DOX-containing diet to induce PELO knockdown. e, Kaplan–Meier survival plot for mice randomized to DOX-containing (n = 5) or standard (n = 6) diet. Data are mean ± s.e.m. of biological replicates: n = 6 for all cell lines except KP4 and GB1, for which n = 7, and IGROV-1, for which n = 3 in a, n = 3 in b, n = 2 for PANFR0127 and PANFR0071 and n = 3 for CCLF_CORE_0001 in c; n = 9 tumours for standard diet and n = 10 for DOX-containing diet in d. Significance was calculated as follows: left-tailed Student’s t-test (a), right-tailed Student’s t-test (b), pairwise log-rank test (e). Representative data are shown from two experiments in a and one experiment in b–e. Experiments were performed twice for a–c and once for d and e. For immunoblots, vinculin was used as loading control.
To confirm that these viability effects were attributable to PELO loss, we studied whether exogenous expression of PELO could rescue cells from CRISPRi-mediated suppression of endogenous PELO. We found that PELO cDNA expression from an EEF1A1 promoter was not affected by the three CRISPRi gRNAs targeting endogenous PELO in MIA PaCa-2 (9p21.3−/−MSS) cells. Correspondingly, PELO cDNA expression rescued MIA PaCa-2 cells from the viability effects of gRNAs targeting PELO, indicating that CRISPRi-associated impairment of viability was due to on-target PELO knockdown (right-tailed Student’s t-test, effect size = 0.675, n = 6, P = 2.10 × 10−6 ; Fig. 2b).
We next sought to validate PELO dependency in tumour organoid models for which we did not have large-scale CRISPR data and for which we could therefore only predict any requirement for PELO on the basis of the two identified biomarkers. Specifically, we interrogated the viability effects of PELO depletion in three patient-derived organoid models representing a 9p21.3+/MSS pancreatic cancer (PANFR0071), 9p21.3−/−/MSS pancreatic cancer (PANFR0127) and an 9p21.3+/MSI-H colorectal cancer (CCLF_CORE_0001). Using CRISPR repressor dCas9–KRAB–MeCP2 with a doxycycline (DOX)-inducible gRNA, we evaluated the effects of DOX-inducible gRNAs targeting Ch2-2, POLR2D or PELO34. We first confirmed that DOX treatment suppressed PELO protein levels in cells with DOX-inducible PELO gRNA 2 (Fig. 2c). PELO depletion substantially impaired the viability of 9p21.3−/−and MSI-H models, to a level similar to that observed with POLR2D knockdown (Fig. 2c). By contrast, PELO depletion in the 9p21.3+/MSS model had no discernible effect on viability, whereas POLR2D depletion still suppressed viability. These data support PELO as a synthetic lethal target in cells with MSI-H or large 9p21.3 deletions.
Next, we sought to evaluate the effects of PELO in tumour maintenance in vivo. We used the DOX-inducible CRISPRi system to suppress PELO in MIA PaCa-2 cells (9p21.3−/−/MSS). We subcutaneously engrafted engineered MIA PaCa-2 cells in the flanks of nude mice. Once the tumours had reached a volume of approximately 150 mm3 (range 50–300 mm3), mice were randomized to a DOX-containing diet or maintained on a standard diet. We verified by immunoblotting that PELO was knocked down as early as 4 days following transition to a DOX-containing diet (Extended Data Fig. 2c). All mice randomized to a standard diet were euthanized within 66 days owing to tumour growth reaching size end points. By contrast, we observed tumour regression in all mice treated with DOX (Fig. 2d and Extended Data Fig. 2d). After all mice in the standard diet cohort had been euthanized, mice in the DOX-treated cohort were reverted to a standard diet to determine whether previously treated tumours would regrow. By 64 days following removal of DOX, we observed that eight of nine tumours remained unchanged or impalpable, suggesting that PELO suppression eradicated most tumour cells. Notably, all mice randomized to DOX were alive 130 days following randomization (including 64 days without DOX), underscoring the sensitivity of these tumours to PELO depletion and showing significant improvement in overall survival (pairwise log-rank test, statistic = 9.71, n = 11, P = 0.002; Fig. 2e).
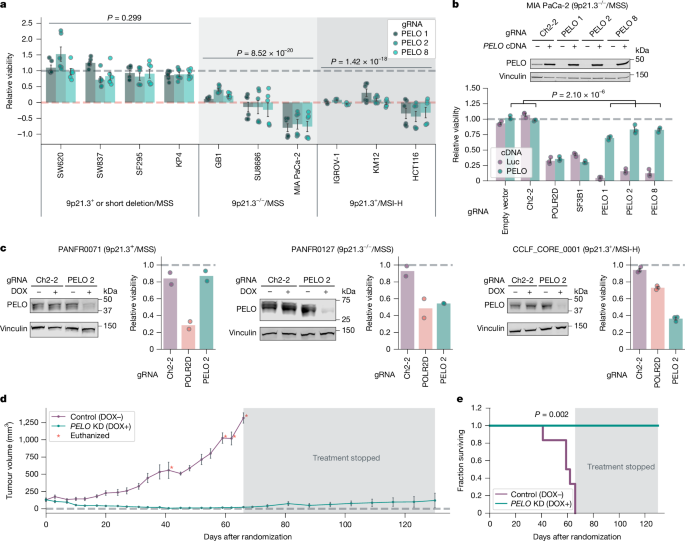
We next pursued the lesion in 9p21.3−/− cancers that confers PELO dependency. On the basis of the hypothesis that loss of a gene on 9p21.3 confers PELO dependency, we analysed DepMap for 9p21.3 genes that were correlated with PELO dependency in 9p21.3−/−cell lines. These data identified several 9p21.3 genes for which loss was similarly and strongly associated with PELO dependency, probably because these genes are frequently codeleted (Extended Data Fig. 3a). We thus performed a loss-of-function CRISPR–enhanced Acidaminococcus sp. Cas12a variant (enAsCas12a) screen with a focused library targeting eight 9p21.3 genes (Extended Data Fig. 3a) in combination with CRISPRi-mediated PELO knockdown35. We used KP4 (9p21.3 short deletion/MSS) cells, in which we saw no significant impairment in viability on PELO depletion (Fig. 2a). We transduced the enAsCas12a 9p21.3 gRNA library in KP4 cells expressing dCas9–KRAB and PELO gRNA 1 and 2, or Ch2-2 control (Fig. 3a). We observed that the mRNA surveillance gene FOCAD was the only gene whose knockout led to preferential impairment in cells with PELO depletion compared with control cells (left-tailed Wilcoxon rank-sum test, effect size = −0.786, n = 552, P = 1.80 × 10−19; Fig. 3a).
a, Left, CRISPR knockout (KO) screen targeting 9p21.3 genes in KP4 cells with CRISPRi knockdown using Ch2-2 gRNA (control), or PELO gRNA 1 or 2. Right, differences in Chronos gene effect scores between PELO and Ch2-2 knockdown for both PELO gRNAs. b, Top, immunoblots of FOCAD, PELO and GAPDH in WM793 cells. Bottom, relative viability of WM793 cells with Ch2 gRNA or FOCAD knockout ± DOX-induced PELO knockdown. c, Top, immunoblots of FOCAD, PELO and α-tubulin in MIA PaCa-2 cells. Bottom, relative viability in MIA PaCa-2 cells stably expressing indicated cDNA ± DOX-induced PELO knockdown. d, Pearson correlation of microsatellite site length with PELO dependency scores in MSI-H cells (n = 73 cell lines). e, Cell lines (n = 1,100) plotted by PELO dependency and length of TTC37 intron 29 microsatellite repeats. f, Top, immunoblots of TTC37, PELO and β-actin in KP4 cells. Bottom, relative viability of KP4 ± TTC37 knockout ± DOX-induced PELO knockdown cells. g, Top, immunoblots of TTC37, PELO and β-actin in HCT116. Bottom, relative viability of HCT116 cells stably expressing the indicated cDNA ± DOX-induced PELO knockdown. Data are mean ± s.e.m. of biological replicates: n = 2 in a; n = 3 in b, f and g; and n = 3 except for PELO cDNA in DOX− condition, for which n = 2, in c. Significance was calculated as follows: in a, left-tailed Wilcoxon rank-sum test; in b and f, left-tailed Student’s t-test; in c and g, right-tailed Student’s t-test. Representative data from one experiment are shown. All experiments were performed twice, except for the experiment in a, which was performed once. For immunoblots, GAPDH, α-tubulin and β-actin were used as loading controls. Neg. ctrl, negative control; Pos. ctrl, positive control.
To evaluate whether FOCAD loss is necessary and/or sufficient for PELO dependency, we engineered three cell lines with or without FOCAD. We first interrogated whether FOCAD deletion was sufficient to confer dependency on PELO for viability. To do this, we knocked out FOCAD in two cell lines, WM793 (9p21.3+/MSS) and KP4 (9p21.3 short deletion/MSS) (Fig. 3b and Extended Data Fig. 3b). In tandem, a DOX-inducible CRISPRi gRNA targeting PELO was introduced into cells. We found that PELO was required for viability in these cell lines only upon FOCAD deletion (left-tailed Student’s t-test, WM793: effect size = −0.636, n = 6, P = 1.99 × 10−7; KP4: effect size = −0.51, n = 9, P = 8.79 × 10−6; Fig. 3b and Extended Data Fig. 3b), demonstrating that FOCAD deletion is sufficient to induce a dependency on PELO. We next investigated whether FOCAD loss was necessary for PELO dependency. For this purpose, we used MIA PaCa-2, a 9p21.3−/−/MSS model with biallelic loss of FOCAD. We exogenously expressed FOCAD, PELO, or control luciferase (Luc) cDNA and suppressed PELO expression with DOX-inducible PELO gRNA 2. In the presence of the control Luc cDNA, MIA PaCa-2 cells remained highly dependent on PELO for survival. By contrast, expression of FOCAD cDNA rescued MIA PaCa-2 viability (right-tailed Student’s t-test, effect size = 0.470, n = 6, P = 0.007; Fig. 3c) to a similar degree to PELO cDNA (right-tailed Student’s t-test, effect size = 0.479, n = 6, P = 0.005). Thus, our data show that FOCAD deletion is both necessary and sufficient for PELO dependency in the 9p21.3−/− context.
Having refined our predictive biomarker for PELO dependency to FOCAD in 9p21.3−/− cancers, we next queried the prevalence of FOCAD copy number in primary tumour samples. Using The Cancer Genome Atlas (TCGA) Pan-Cancer dataset, we found that FOCAD was deeply deleted in 335 out of 10,715 (3.1%) samples with consensus homozygous deletion calls36 (Extended Data Fig. 3c). In this context, biallelic FOCAD deletion is a relatively frequent cancer alteration, occurring more frequently than putative driver EGFR mutations (1.8%, 192 out of 10,443 samples; Extended Data Fig. 3c).
We next sought to determine the mechanistic basis for PELO dependency in the MSI-H context. We proposed the hypothesis that MSI-H-associated mutations in one or more genes sensitize cells to PELO depletion. Hence, we correlated PELO dependency with insertion–deletion mutations common in MSI-H cancers by inferring repeat length per microsatellite with MSIsensor2 for each cell line28 (Fig. 3d). PELO dependency was most strongly correlated with mutations in a microsatellite from chr. 5: 95507014–95507025 (Pearson’s R = 0.656, n = 73, q = 8.49 × 10−7), a sequence of 11 thymidines in the splicing acceptor site at intron 29 of the SKIc member TTC37 (also known as SKIC3). We observed that deletions of thymidines in this microsatellite corresponded with increased PELO dependency in MSI-H cell lines (Fig. 3e). We also noted that TTC37 expression scored as a potential biomarker in our univariate analysis that identified MSI-H score (Fig. 1e), so we reasoned that deletions at this site might alter splicing and destabilize TTC37 mRNA. We found that nearly all cell lines with two or more thymidine deletions in the TTC37 intron 29 polythymidine track were MSI-H, expressed lower levels of the dominant protein-coding transcript (ENST00000358746) and higher levels of an alternative non-coding transcript (ENST00000508181), and possessed reduced TTC37 protein levels37 (Extended Data Fig. 3d,e). These data indicate that TTC37 intron 29 polythymidine deletions might be responsible for the reduced TTC37 protein levels, a finding that will need to be confirmed with future functional studies. We also observed that TTC37 mutations more specifically predicted PELO dependency in FOCAD-intact cell lines than MSI-H status alone (Fig. 1d and Extended Data Fig. 3f).
To evaluate the role of TTC37 loss in PELO synthetic lethality, we studied whether TTC37 loss was sufficient and/or necessary for PELO dependency in MSI-H cells. First, we knocked out TTC37 with CRISPR–enAsCas12 in KP4 cells (9p21.3 short deletion/MSS). We then treated cells with DOX to induce a CRISPRi gRNA targeting PELO. We observed that PELO knockdown impaired the viability of TTC37-deleted but not control KP4 cells (left-tailed Student’s t-test, effect size = −0.433, n = 9, P = 4.55 × 10−4; Fig. 3f). These data demonstrate that TTC37 loss is sufficient to confer a dependency on PELO for survival. We next asked whether TTC37 loss was necessary for PELO dependency in the MSI-H context. We exogenously expressed TTC37 or Luc cDNA in three MSI-H colorectal cancer cell lines, HCT116, KM12 and DLD1, all containing a DOX-inducible PELO knockdown system. We found that exogenous expression of TTC37 but not Luc rescued the viability of all three MSI-H cell lines from PELO knockdown (right-tailed Student’s t-test; HCT116: effect size = 0.505, n = 6, P = 3.2 × 10−5; KM12: effect size = 0.492, n = 6, P = 5.70 × 10−7; DLD1: effect size = 0.407, n = 6, P = 9.98 × 10−7; Fig. 3g and Extended Data Fig. 3g,h). Together, these data demonstrate that functional TTC37 impairment is sufficient and necessary for PELO dependency in the MSI-H setting and could serve as a refined predictive biomarker for PELO dependency.
Next, we compared the relationships between our proposed biomarkers and PELO dependency with well-characterized dependency–biomarker relationships. These analyses showed the differential PELO dependency between biomarker-positive (FOCAD deletion or TTC37 mutations) and biomarker-negative cell lines to be comparable with the relationships between KRAS dependency and KRAS mutations or BRAF dependency and BRAF mutations. Similarly, PELO biomarker-positive cell lines were significantly more dependent on PELO than non-cancerous immortalized cell lines (left-tailed Student’s t-test, effect size = −0.981, n = 75 PELO biomarker-positive cell lines, n = 4 non-cancerous cell lines, P = 0.001; Extended Data Fig. 3i).
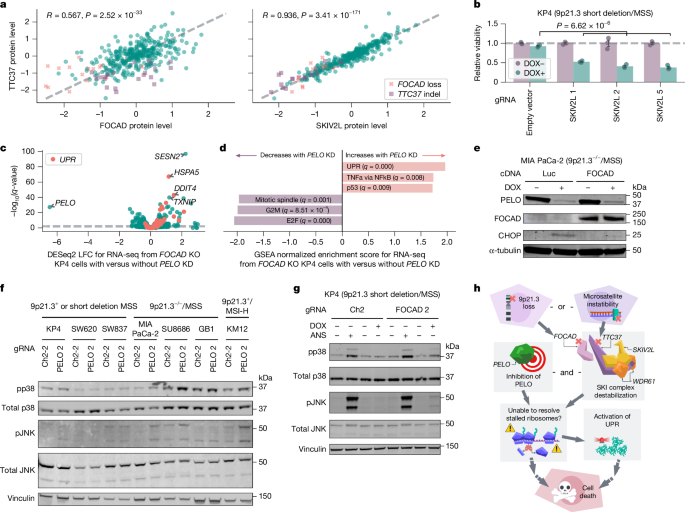
We next sought to investigate how FOCAD loss and TTC37 mutations might converge to confer PELO dependency. TTC37 interacts with SKIV2L and WDR61 to form the SKIc, which promotes 3′→5′ exosome degradation of mRNA from stalled ribosomes38. Notably, FOCAD has been reported to physically interact with and stabilize the SKIc39. Thus, we proposed that protein levels of FOCAD, TTC37, SKIV2L and WDR61 would be correlated. Analyses of the Cancer Cell Line Encyclopedia (CCLE) proteomics dataset demonstrated positive correlations between FOCAD, TTC37 and SKIV2L protein levels37 (TTC37 and FOCAD: Pearson’s R = 0.567, n = 375, P = 2.52 × 10−33; TTC37 and SKIV2L: R = 0.936, n = 375, P = 3.41 × 10−171; SKIV2L and FOCAD: R = 0.553, n = 375, P = 1.86 × 10−31; Fig. 4a, Extended Data Fig. 4a). WDR61 protein levels showed little to no correlation with levels of the other three proteins (Extended Data Fig. 4a); this may reflect the dual role of WDR61 as a subunit of both the SKIc and the (PAF complex37,40. We examined FOCAD, TTC37 and SKIV2L (also known as SKIC2) mRNA expression in DepMap and observed little correlation (Extended Data Fig. 4a). There was also no significant correlation between FOCAD protein levels and TTC37 or SKIV2L mRNA expression, concordant with reports that FOCAD–SKIc interactions occur on the protein level39,41 (Extended Data Fig. 4b). Consistent with CCLE proteomic data, we observed decreased TTC37 and SKIV2L protein levels in 9p21.3−/−/MSS and 9p21.3+/MSI-H models with immunoblotting (Extended Data Fig. 4c,d). On the basis of these results, we asked whether FOCAD was required and/or sufficient to maintain TTC37 and SKIV2L protein stability. We exogenously expressed FOCAD cDNA in MIA PaCa-2 cells (9p21.3−/−/MSS) and observed increased TTC37 and SKIV2L protein levels (Extended Data Fig. 5a). In addition, we found that FOCAD knockout reduced SKIV2L and TTC37 protein levels in KP4 cells (9p21.3 short deletion/MSS) (Extended Data Fig. 5b). Together, these data support the claim that FOCAD is a crucial regulator of SKIV2L and TTC37 stability39,41.
a, Comparison of TTC37, FOCAD and SKIV2L protein levels across cell lines (n = 375) with FOCAD loss (relative copy number < 0.4, n = 17) or TTC37 insertion–deletion (indel; ≤9 microsatellite repeats, n = 23) indicated. b, Relative viability of KP4 cells with the indicated CRISPR knockout gRNAs ± DOX-induced PELO knockdown. c, q-values from DESeq2 Wald test for differential expression between RNA sequencing from KP4 FOCAD knockout cells ± DOX-induced PELO knockdown plotted against log2-transformed fold change. Genes in the Hallmark UPR gene set are highlighted. d, Hallmark gene set enrichment prerank results for log2-transformed fold changes shown in c. e, Immunoblots of PELO, FOCAD, CHOP and α-tubulin in MIA PaCa-2 cells with Luc or FOCAD cDNA ± DOX-induced PELO knockdown. f, Immunoblots of phospho-p38 (pp38, Thr180/Tyr182), total p38, phospho-JNK (pJNK, Thr183/Tyr185), total JNK and vinculin in the indicated cell lines with Ch2-2 gRNA or PELO knockdown. g, Immunoblots of pp38 (Thr180/Tyr182), total p38, pJNK (Thr183/Tyr185), total JNK and vinculin for KP4 cells with Ch2 gRNA or FOCAD knockout ± DOX-induced PELO knockdown ± anisomycin (ANS, positive control). h, Model of synthetic lethality between PELO dependency and 9p21.3−/− and MSI-H cancers. Created in Lucid (lucid.co). Data are mean ± s.e.m. of biological replicates: n = 3 for (b–d). Significance was calculated using right-tailed Student’s t-test in b. Representative data from one experiment are shown. All experiments were performed twice, except for experiments in c,d,f, which were performed once. For immunoblots, α-tubulin and vinculin were used as loading controls.
These data suggest that both FOCAD deletions and TTC37 mutations capture SKIc loss of function, thereby conferring increased reliance on PELO for survival. If functional loss of the SKIc was responsible for heightened PELO dependency, we proposed that SKIV2L deletion would confer increased PELO dependency. To investigate this, we knocked out SKIV2L with enAsCas12a gRNAs in KP4 cells (9p21.3 short deletion/MSS) and found depletion of SKIV2L with all three gRNAs (Extended Data Fig. 5c). In contrast to SKIV2L-intact KP4 cells, DOX-mediated PELO knockdown markedly impaired the viability of SKIV2L knockout KP4 cells (right-tailed Student’s t-test, effect size = 0.489, n = 12, P = 6.62 × 10−6; Fig. 4b). We also found that the minimum protein level of the three SKI complex members (TTC37, SKIV2L and WDR61) was highly predictive of PELO dependency (Pearson’s R = 0.514, n = 302, P = 8.90 × 10−22; Extended Data Fig. 5d). Similarly, PELO dependency could be predicted by SKIV2L protein levels alone (Pearson’s R = 0.49, n = 302, P = 1.18 × 10−19; Extended Data Fig. 5e), supporting our hypothesis that loss of SKIc function confers increased dependency on PELO for survival.
To assess how SKIc-deficient cancer cells responded to PELO suppression, we performed gene expression profiling. We observed minimal transcriptional responses following FOCAD knockout or PELO knockdown in parental KP4 cells (Extended Data Fig. 5f,g). By contrast, PELO knockdown resulted in a robust transcriptional response in FOCAD-deleted KP4 cells, with gene set enrichment analysis demonstrating upregulation of the unfolded protein response (UPR), a signalling network that responds to aggregated misfolded proteins42,43,44 (gene set enrichment analysis prerank, normalized enrichment score = 1.96, q = 0.000; Fig. 4c,d).
To validate these findings, we performed immunoblotting and found that PELO knockdown increased levels of UPR marker C/EBP homologous protein (CHOP) in 9p21.3−/−/MSS and 9p21.3+/MSI-H cells but not 9p21.3+/MSS cells (Extended Data Fig. 5h). We also found that restoring or destabilizing SKIc modulated UPR activation following PELO knockdown. FOCAD cDNA expression reduced CHOP levels in MIA PaCa-2 cells (9p21.3−/−/MSS) following PELO knockdown (Fig. 4e). In addition, CHOP levels and XBP1 mRNA splicing, which are markers of UPR activation, increased upon PELO knockdown in FOCAD-deleted (but not FOCAD-intact) KP4 cells45 (right-tailed Student’s t-test, effect size = 0.821, n = 6, P = 3.76 × 10−4; Extended Data Fig. 5i,j). These data demonstrate that PELO depletion preferentially activates the UPR in the SKIc-deficient context. In addition to dissociating stalled ribosomes, the PELO–HBS1L complex has been shown to promote activation of ribosome-associated quality control, a process that degrades the nascent polypeptides that remain bound to 60S subunits after ribosomal splitting46. Thus, we propose a model in which loss of SKIc function to extract mRNA from stalled ribosomes uncovers an increased dependency on PELO–HBS1L to recruit ABCE1 and split the ribosome. In the SKIc-deficient context, loss of PELO–HBS1L activity to split ribosomes and activate ribosome-associated quality control may lead to accumulation of nascent polypeptides, activating the UPR.
UPR and other pathways responding to ribosomal stress, including the ribotoxic stress response, have been demonstrated to activate stress- and mitogen-activated proteins p38 and JNK47,48,49,50,51,52. Here, we evaluated whether PELO depletion activated the p38/JNK stress response in SKIc-deficient cells. We performed immunoblotting for phospho-p38 (Thr180/Tyr182) and phospho-JNK (Thr183/Tyr185), markers of p38 and JNK activation, respectively, across various cell lines representing 9p21.3+ or short deletion/MSS, 9p21.3−/−/MSS or 9p21.3+/MSI-H (Fig. 4f). We observed that PELO depletion preferentially induced both p38 and JNK phosphorylation in nearly all cell lines predicted to be SKIc deficient. In addition, FOCAD knockout in KP4 (9p21.3 short deletion/MSS) cells led to p38 and JNK phosphorylation following PELO depletion, mirroring the observed viability effects (Fig. 4g and Extended Data Fig. 3b). These data show that PELO loss and impairment of SKIc synergize to activate multiple stress pathways including the UPR and the p38/JNK stress response.
Our observations reveal that MSI-H associated mutations and large 9p21.3 deletions involving TTC37 and FOCAD, respectively, independently impair the SKIc and confer a synthetic lethal relationship with PELO (Fig. 4h). As MSI-H and large 9p21.3 deletions are frequently observed in patients, a PELO-based therapeutic could have broad implications for clinical oncology. Although complete loss of PELO is embryonically lethal in mice, our data suggest that there is an exploitable therapeutic window with PELO inhibition53. Our data also support the development of predictive biomarkers based on SKIc protein detection, such as immunohistochemistry staining for SKIc subunits, to select patients who would be most likely to benefit from PELO-based therapeutics. Further studies will also be needed to explore the intersecting roles of PELO and the SKIc in mammalian cells. Notably, SKI2, the yeast homologue of SKIV2L has been functionally linked with Dom34, the yeast homologue of PELO, as both have been reported to help resolve stalled ribosomes54. Studies in Saccharomyces cerevisiae and Caenorhabditis elegans suggest that their PELO and SKIc homologues interact to resolve stalled ribosomes through recursive rounds of non-stop decay, particularly when a ribosome translates and arrests at the 3′ end of mRNA55. These models suggest that following endonuclease cleavage at the 5′ edge of the ribosome, the downstream stalled ribosome is rescued by PELO, while the SKIc clears the 3′ tail of the upstream mRNA fragment54,56,57,58. However, PELO and SKIc homologues are not synthetic lethal in S. cerevisiae, suggesting mechanistic differences in humans59. In C. elegans, the synthetic lethal relationship is nuanced and incomplete, with double SKIc and PELO homologue mutants exhibiting impaired fertility that is modulated by temperature58. Subsequent efforts will need to investigate why such a robust synthetic lethal phenotype arises in human cells, as well as the ribotoxic lesions that confer a requirement for PELO and/or SKIc and the downstream effectors that signal cell death. More broadly, our findings underscore the potential of DepMap to uncover novel therapeutic targets and functional interactions, opening new avenues for therapeutic interventions and fundamental biological understanding.