Fu, Q. et al. Interface-confined ferrous centers for catalytic oxidation. Science 328, 1141–1144 (2010).
Cao, L. et al. Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2. Nature 565, 631–635 (2019).
Ye, T.-N. et al. Vacancy-enabled N2 activation for ammonia synthesis on an Ni-loaded catalyst. Nature 583, 391–395 (2020).
Lin, L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80–83 (2017).
Cargnello, M. et al. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 341, 771–773 (2013).
van Deelen, T. W., Mejia, C. H. & de Jong, K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955–970 (2019).
Ro, I. et al. Bifunctional hydroformylation on heterogeneous Rh-WOx pair site catalysts. Nature 609, 287–292 (2022).
Liu, Z. et al. Water-promoted interfacial pathways in methane oxidation to methanol on a CeO2-Cu2O catalyst. Science 368, 513–517 (2020).
Zhang, X. et al. A stable low-temperature H2-production catalyst by crowding Pt on α-MoC. Nature 589, 396–401 (2021).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Peng, M. et al. Fully exposed cluster catalyst (FECC): toward rich surface sites and full atom utilization efficiency. ACS Central Sci. 7, 262–273 (2021).
Dong, C. Y. et al. Fully exposed palladium cluster catalysts enable hydrogen production from nitrogen heterocycles. Nat. Catal. 5, 485–493 (2022).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Hannagan, R. T. et al. First-principles design of a single-atom-alloy propane dehydrogenation catalyst. Science 372, 1444–1447 (2021).
Hulva, J. et al. Unraveling CO adsorption on model single-atom catalysts. Science 371, 375–379 (2021).
Cargnello, M. et al. Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 337, 713–717 (2012).
Li, X. et al. Functional CeOx nanoglues for robust atomically dispersed catalysts. Nature 611, 284–288 (2022).
Yang, X. et al. Taming the stability of Pd active phases through a compartmentalizing strategy toward nanostructured catalyst supports. Nat. Commun. 10, 1611 (2019).
Peterson, E. J. et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 5, 4885 (2014).
Levy, R. B. & Boudart, M. Platinum-like behavior of tungsten carbide in surface catalysis. Science 181, 547–549, (1973).
Chen, J. G. G. Carbide and nitride overlayers on early transition metal surfaces: preparation, characterization, and reactivities. Chem. Rev. 96, 1477–1498 (1996).
Hwu, H. H. & Chen, J. G. Surface chemistry of transition metal carbides. Chem. Rev. 105, 185–212 (2005).
Lin, L. L. et al. Atomically dispersed Ni/α-MoC catalyst for hydrogen production from methanol/water. J. Am. Chem. Soc. 143, 309–317 (2021).
Setthapun, W., Bej, S. K. & Thompson, L. T. Carbide and nitride supported methanol steam reforming catalysts: Parallel synthesis and high throughput screening. Top. Catal. 49, 73–80 (2008).
Yao, S. et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction. Science 357, 389–393 (2017).
Zhang, Z.-S. et al. Intrinsically active surface in a Pt/γ-Mo2N catalyst for the water-gas shift reaction: molybdenum nitride or molybdenum oxide? J. Am. Chem. Soc. 142, 13362–13371 (2020).
Hansen, T. W., DeLaRiva, A. T., Challa, S. R. & Datye, A. K. Sintering of catalytic nanoparticles: particle migration or Ostwald ripening? Acc. Chem. Res. 46, 1720–1730, (2013).
Zheng, X., Lin, H., Zheng, J., Duan, X. & Yuan, Y. Lanthanum oxide-modified Cu/SiO2 as a high-performance catalyst for chemoselective hydrogenation of dimethyl oxalate to ethylene glycol. ACS Catal. 3, 2738–2749 (2013).
Villars, P. (ed.) PAULING FILE in: Inorganic Solid Phases, SpringerMaterials c_0210243 (Springer-Verlag, 2016); https://materials.springer.com/isp/phase-diagram/docs/c_0210243.
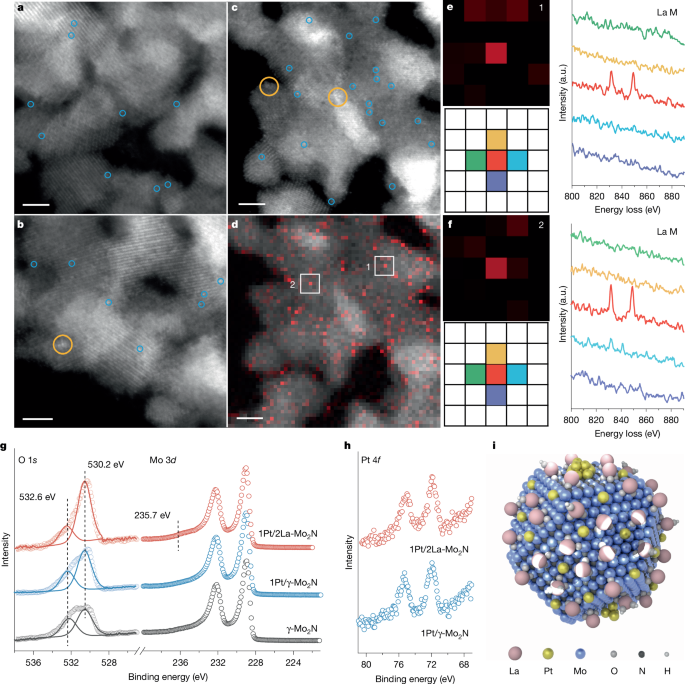
Gao, Z., Li, A., Ma, D. & Zhou, W. Electron energy loss spectroscopy for single atom catalysis. Top. Catal. 65, 1609–1619 (2022).
Haynes, W. M. (ed.) CRC Handbook of Chemistry and Physics 92nd edn (CRC Press, 2011).
Nielsen, M. et al. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 495, 85–89 (2013).
Ziegler, C. et al. ZnPd/ZnO aerogels as potential catalytic materials. Adv. Funct. Mater. 26, 1014–1020 (2016).
Cortright, R. D., Davda, R. R. & Dumesic, J. A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 418, 964–967, (2002).
Tian, H., Roberts, C. A. & Wachs, I. E. Molecular structural determination of molybdena in different environments: aqueous solutions, bulk mixed oxides, and supported MoO3 catalysts. J. Phys. Chem. C 114, 14110–14120 (2010).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 48, 13115–13118 (1993).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A Consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104–154123 (2010).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA + U study. Phys. Rev. B 57, 1505–1509 (1998).
Henkelman, G., Uberuaga, B. P. & Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Shao, Z. et al. Maximizing the synergistic effect between Pt0 and Ptδ+ in a confined Pt-based catalyst for durable hydrogen production. Appl. Catal. B Environ. 316, 121669 (2022).
Gupta, P., Dwivedi, S., van Duin, A. C. T., Srinivas, S. & Tanksale, A. Coke resistant catalyst for hydrogen production in a versatile, multi-fuel, reformer. J. Catal. 402, 177–193 (2021).
Köwitsch, N. et al. Unprecedented catalytic activity and selectivity in methanol steam reforming by reactive transformation of intermetallic In–Pt compounds. J. Phys. Chem. C 125, 9809–9817 (2021).
Wang, H. et al. Titanosilicate zeolite supported Pt nanoparticles with electronic metal-support interactions for efficient methanol steam reforming. Catal. Today 382, 42–47 (2021).
Liao, L. et al. Unravelling the morphology effect of Pt/In2O3 catalysts for highly efficient hydrogen production by methanol steam reforming. Fuel 372, 132221 (2024).
Li, D., Sun, J., Ma, R. & Wei, J. High-efficient solar-driven hydrogen production by full-spectrum synergistic photo-thermo-catalytic methanol steam reforming with in-situ photoreduced Pt-CuOx catalyst. J. Energy Chem. 71, 460–469 (2022).
Wang, Y., Yao, E.-P., Wu, L., Feldmann, J. & Stolarczyk, J. K. A multi-layer device for light-triggered hydrogen production from alkaline methanol. Angew. Chem. Int. Ed. 60, 26694–26701 (2021).
Luo, J. et al. Efficient base-free aqueous reforming of methanol homogeneously catalyzed by ruthenium exhibiting a remarkable acceleration by added catalytic thiol. J. Am. Chem. Soc. 143, 17284–17291 (2021).
Hu, P., Diskin-Posner, Y., Ben-David, Y. & Milstein, D. Reusable homogeneous catalytic system for hydrogen production from methanol and water. ACS Catal. 4, 2649–2652 (2014).
Fujita, K., Kawahara, R., Aikawa, T. & Yamaguchi, R. Hydrogen production from a methanol-water solution catalyzed by an anionic iridium complex bearing a functional bipyridonate ligand under weakly basic conditions. Angew. Chem. Int. Ed. 54, 9057–9060 (2015).
Alberico, E. et al. Selective hydrogen production from methanol with a defined iron pincer catalyst under mild conditions. Angew. Chem. Int. Ed. 52, 14162–14166 (2013).
Bielinski, E. A. et al. Base-free methanol dehydrogenation using a pincer-supported iron compound and Lewis acid co-catalyst. ACS Catal. 5, 2404–2415 (2015).