Retrospective studies
We completed a non-interventional, retrospective review of patient data using the MDACC electronic health record system, which contains a record of the patients who are treated at the primary campus of MDACC, a large quaternary cancer hospital in Houston, Texas. The chart review for this study involved three groups of patients: (1) patients with tumour biopsies confirming stage III or stage IV NSCLC between January 2017 and September 2022; (2) patients with melanoma of any stage who received single- or multi-agent immune checkpoint blockade between January 2019 and December 2022; and (3) a tissue-agnostic cohort, which included all patients with pathology results for PD-L1 from January 2020 to October 2023 at our institution across a wide range of histologies. This study was approved by the MD Anderson Cancer Center institutional review board. Informed consent was waived due to the retrospective and de-identified nature of the data. The data-collection cut-off was 1 September 2024; data analysis was performed from 1 September 2024 to 29 July 2025.
In the NSCLC dataset, patient information was collected regarding patient demographics (such as age at immunotherapy start, sex, ethnicity), primary tumour histology, clinical stage, known tumour mutations (such as those in EGFR, KRAS, HER2, ALK, MET, p53 and RET), metastatic burden at immunotherapy start (brain, liver), Eastern Cooperative Oncology Group (ECOG) performance status (PS; range, 0–5) near the initiation of immunotherapy, radiation therapy in the time around immunotherapy start, chemotherapy history, immunodeficiency, comorbidities (heart disease, kidney disease, liver disease, respiratory disease), history of other primary tumours, steroid use around immunotherapy initiation, date of last follow-up, date of death, date of first recurrence or progression, ICI agent names and start dates, COVID-19, influenza and pneumococcal vaccination dates, and TPS. Recorded PD-L1 expression as reported below was required for inclusion in the survival analysis for NSCLC. For patients with multiple biopsies, the PD-L1 expression recorded from the closest biopsy to ICI start was used for analysis.
In the melanoma dataset, patient information was collected regarding patient demographics (including age at immunotherapy start, sex, ethnicity), primary tumour histology, clinical stage, known tumour mutations (such as those in EGFR, KRAS, HER2, ALK, MET, p53 and RET), metastatic burden at immunotherapy start (for example, brain, liver), PS (range, 0–5) at the initiation of immunotherapy, chemotherapy history, immunodeficiency, comorbidities (for example, heart disease, kidney disease, liver disease, respiratory disease), other primary tumour data, steroid use around COVID-19 vaccination and immunotherapy initiation, date of last follow-up, date of death, date of first recurrence/progression, ICI agent names and start dates, and COVID-19 vaccination dates. Although our dataset did not include specific vaccine formulations administered to each patient, vaccine formulations administered during the study period included the original monovalent mRNA-1273 vaccine from Moderna (100 µg mRNA prime, 50 µg mRNA booster) released on 18 December 2020; the bivalent Moderna vaccine targeting the original strain and Omicron BA.4/BA.5 (50 µg mRNA) released on 1 September 2022; the original monovalent vaccine from Pfizer/BioNTech (30 µg mRNA prime and booster) released on 11 December 2020; and the Pfizer/BioNTech bivalent formulation (30 µg mRNA) released on 31 August 2022.
Patients were separated into two groups: (1) patients who received a COVID-19 mRNA vaccination within 100 days of ICI start; and (2) patients who did not receive a COVID-19 vaccination. Survival analysis was performed using these groups, with subanalysis involving staging of the tumour, brand of mRNA vaccine, number of doses of the COVID-19 vaccine, location of metastases and cycle of immunotherapy.
For patients who received COVID-19 mRNA vaccination in both the NSCLC and melanoma datasets, OS was calculated as the time between the date of immunotherapy start closest to the mRNA vaccination date, and the last follow-up date or date of death. For patients who did not receive COVID-19 mRNA vaccination, OS was calculated as the time between the initiation of their first ICI start and the date of death or last follow-up. For patients who received COVID-19 mRNA vaccination, PFS was calculated as the time between the initiation of ICI closest to mRNA vaccination and the first incidence of either pathology-confirmed recurrence or imaging-confirmed progression, whichever occurred earlier, that was declared progression in their primary medical oncologist’s clinical notes. For patients who did not receive COVID-19 mRNA vaccination, PFS was calculated as the time between their first ICI start date and clinician-confirmed progression as described above. Patients who progressed before the receipt of mRNA vaccination were included in the vaccination group for this analysis. Kaplan–Meier curves were generated using GraphPad Prism.
Cox proportional hazards regression
For Cox proportional hazards regression, time-dependent variables were defined as described in the figure captions. Continuous/numbered variables (such as age, BMI, PD-L1 expression, ECOG and treatment year) were retained as numeric. Binary and categorical variables (for example, stage, gender, mutational status, comorbidities) were converted into factors. For each variable, we constructed individual Cox proportional hazards models. HRs, 95% CIs (Wald) and P values were extracted from model summaries. Variables with P < 0.05 in univariable analysis were considered for multivariable modelling. Multivariable Cox proportional hazard regressions were similarly generated with significant variables from univariable analysis. For multivariable Cox proportional hazards regressions, patients with missing values were excluded from analyses.
Categories with fewer than five cases are reported for completeness but were considered not statistically meaningful due to convergence and insufficient data for reliable inference. Variables with significant relationships with survival were included in multivariable analyses (MVAs), and those significant after MVA (P < 0.05) were included in PSM. Certain variables (for example, steroid use within 1 month of vaccine) represented subgroups of the treatment cohort by definition. These were evaluated descriptively and in univariable models but were not included as covariables in MVAs, as they do not represent baseline confounders and were not significant on univariable analysis.
We assessed the proportional hazards assumption in multivariate models by evaluating Schoenfeld residuals and found no major violations.
Imputation and PSM
A small number of patients in the NSCLC dataset did not have documented ECOG scores, which were an important predictor of outcomes in Cox proportional hazards regression. BMI had missingness in initial univariable analyses but, because it was not required for PSM, imputation was not performed to address the missingness. Multivariable logistic regression and ridge regression were used to evaluate associations between ECOG missingness and clinically relevant pretreatment covariables. Ridge regression was selected due to multicollinearity between important variables that could not be excluded from the model. Variables not included in models predicting ECOG missingness were vaccination status, steroid usage within a timeframe of vaccination or ICI, PD-L1, concurrent chemotherapy, immunotherapy agent and BMI (as mentioned above). Subsequent ridge regression analysis suggested that ECOG scores in this analysis had a high probability of exhibiting missingness at random.
Given the small sample size in the stage III NSCLC dataset treatment group, multiple imputation was performed to estimate missing ECOG values using chained equations and the R package MICE, where predictive mean matching (PMM) (with set.seed(2025)) was used to create five imputed datasets. Variables used for imputation were chosen based on large absolute ridge regression coefficients (|β| > 0.3) and clinical relevance to ensure that prediction of ECOG missingness occurred with accuracy. After selection using regression coefficients, age and previous cycles of systemic therapy were included due to clinical importance. To facilitate PSM while maintaining power in patients with stage III NSCLC, the mode of the five imputed ECOG values was used as a single value for each patient (n = 5). This approach was necessary to minimize variance inflation and avoid diluting treatment effects in a very small sample. As PMM is random, small deviations in variable definition, factor levels and row ordering may result in slightly different imputed values. However, sensitivity analyses with slightly different mode selection in the case of ties, different seeds and an increased number of datasets (n = 20 or n = 10 rather than n = 5) revealed similar outcomes (P < 0.05) after subsequent matching. The plausibility of imputed values was assessed using density plots, which demonstrated appropriate range and overlap between observed and imputed ECOG values.
After imputation, PSM was performed using the R MatchIt package. A logistic regression model was used to estimate propensity scores, predicting treatment assignment of ‘vaccine within 100 days’ or ‘no vaccine’ with covariables based on significant factors associated with survival from MVA, including original or imputed ECOG scores. Across all propensity score analyses, nearest-neighbour matching was performed on the previously estimated propensity scores to identify a balanced cohort. In the case of stage III unresectable NSCLC PSM, nearest-neighbour PSM was performed with a caliper of 0.1 and a 2:1 ratio of control patients to treated patients in stage III UR NSCLC to improve covariable balance and maximize statistical power. Sensitivity analysis with removal of ECOG from the PSM model revealed similar trends.
In stage IV NSCLC, patients with missing ECOG were excluded due to sufficient remaining sample size. PSM was once again performed with nearest-neighbour matching, with a caliper of 0.1 and a 1:1 ratio of control patients to treated patients. Sensitivity analyses with removal of ECOG from PSM rather than imputation and with the same methods as reported in stage III NSCLC (with the same caliper of 0.1 but with a ratio of 1:1 rather than 2:1) also each revealed statistically significant results.
No imputation was performed in the melanoma cohort due to completeness of variables. The melanoma PSM analyses were run with nearest-neighbour matching with a caliper of 0.1 and a 1:1 ratio of control patients to treated patients.
Across all matched cohorts, absolute standardized mean differences (|SMD|) of pscores were consistently less than 0.05 after matching, indicating excellent balance between groups. Balance diagnostics were confirmed visually using jitter plots and histograms of propensity scores.
Survival analysis
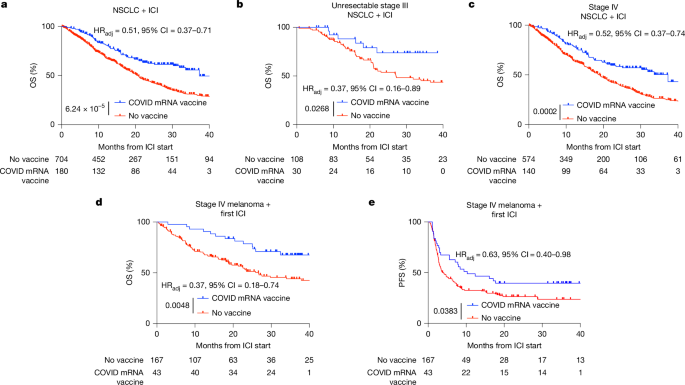
The Kaplan–Meier method was used to estimate survival distributions for the matched groups. Log-rank tests were conducted to assess the differences between treatment groups. GraphPad Prism was used to visualize Kaplan–Meier curves, 95% CIs and at-risk numbers at 0, 10, 20, 30 and 40 months, including after extraction of propensity score-matched data from R. Comparisons between groups in primary datasets (Fig. 1) were reported as adjusted HRs calculated with Cox proportional hazard regression. Comparisons between groups in subgroup analyses were reported as unadjusted HRs calculated with log-rank tests to preserve robustness. P values are reported for primary survival analyses.
In settings in which Kaplan–Meier curves crossed, the restricted mean survival time was calculated using area under the curve analysis of the absolute differences between arms at 12 and 24 months.
All statistical analyses were conducted in R v.4.4.2 (2024-10-31) and GraphPad Prism.
Quantification of PD-L1 expression in biopsy samples
For patients in both the tissue-agnostic cohort and the NSCLC dataset, biopsy date, pathology reports, histological information and diagnosis codes were collected for patients who had received a biopsy with pathology evaluating PD-L1 at MD Anderson (from January 2020 to October 2023 for the tissue-agnostic cohort and August 2016 to August 2022 for the NSCLC cohort). PD-L1 on tumour cells (TPS) and PD-L1 on tumour cells and immune cells together (CPS) were obtained from pathology reports. Data were curated according to the following principles: (1) simple quantitative values were replicated exactly; (2) TPS values of “<1%” were reported as 0%; (3) for patients with a CPS of 0% or CPS <1% with no TPS value, TPS was reported as 0%, as CPS of 0 or <1% implies a lack of expression of PD-L1 on both tumour cells and myeloid cells; (4) TPS values between 1.1 and 49.9 reported as “<x%” or “>x%” were excluded due to lack of interpretability; (5) TPS values of >50% were recorded as 50% and TPS values >60% were recorded as 60%, and so on, as these values indicate high PD-L1 expression. For ranges of PD-L1 expression (for example, 0–5%), the median of the numbers in the range was taken as an approximate estimation of PD-L1 on tumours. Replicate patient biopsies (repeat biopsies performed on the same patient within 2 months of a previous biopsy) were excluded, and the earliest biopsy was included.
Separately, survival was analysed in the tumour-agnostic dataset with any COVID-19 mRNA vaccine within 100 days of ICI compared with patients who had no recorded history of a COVID-19 vaccine. Survival analysis was performed as listed in the NSCLC dataset for all patients in this dataset who were treated with ICI in this cohort. TPS values were not required for inclusion.
For group-wise analysis, patients in these cohorts were grouped into (1) those who received their closest COVID-19 mRNA vaccination less than 100 days before tissue biopsy; (2) patients who received their closest COVID-19 mRNA vaccine more than or exactly 100 days before tissue biopsy; and (3) patients who did not receive a COVID-19 mRNA vaccination before biopsy. Patients who received non-mRNA-based COVID-19 vaccines within 100 days before biopsy were included in the no-vaccine group.
Healthy human participants
Studies in healthy volunteers were approved by the MD Anderson Institutional Review Board. Informed consent was obtained from each healthy participant before plasma and peripheral blood mononuclear cells were collected and stored at baseline, 6 h, 24 h and 48 h, or baseline, 6 h, 24 h, 7 days and 14 days after COVID-19 mRNA vaccination, depending on the formulation used. BNT162b2 (COVID-19 mRNA vaccine, 2024–2025 formula, with 30 μg mRNA) and mRNA-1273 (Spikevax Monovalent XBB.1.5, COVID-19 mRNA vaccine, 2023–2024 formulation, with 50 μg mRNA) were used for the first and second timeframes, respectively. Frozen plasma was thawed and analysed using the NULISAseq Inflammation Panel 250 by Alamar Biosciences. Absolute quantification of IFNα concentrations was performed using the NULISAseq Inflammation Panel AQ. Statistical analysis was performed using R, in which we evaluated significance using a linear model with fixed effects based on the time from vaccine and controlling for patient ID (paired), including accommodation of biological variance between the baselines using the limma package’s duplicateCorrelation function. P values were calculated using moderated t-tests with FDR correction for multiple testing. For comparisons between Pfizer and Moderna vaccines, we used a linear modelling approach (limma) to assess the log2-transformed fold change in cytokine concentrations per-patient from the baseline to 6 h and 24 h. Moderated t-tests with empirical Bayes shrinkage were applied, and P values were corrected using the Benjamini–Hochberg FDR method. Contrasts were constructed to directly compare Moderna versus Pfizer for each timepoint. Cytokines were visualized if they demonstrated significant differences between Moderna and Pfizer from the baseline to 6 h or 24 h (absolute value of log2-transformed fold change greater than 0.5 and FDR-corrected P < 0.05). Mean expression values per condition were normalized to the baseline and visualized. Volcano plots were generated using R. Heat maps and graphs of cytokines with significant changes in relative expression in the plasma at 24 h were generated in GraphPad Prism.
Human flow cytometry
Peripheral blood mononuclear cells were thawed in a 37 °C water bath and immediately transferred into a 50 ml tube containing 9 ml of complete RPMI medium (RPMI + 10% FBS) at a ratio of 1 part cells to 9 parts medium. Cells were centrifuged at 400g for 10 min, and the supernatant was decanted. The cell pellet was resuspended in 1 ml of PBS, and incubated with live/dead marker (1 µl per 1,000 µl of cell suspension, containing 1–10 million cells per ml) for 30 min at 4 °C. After incubation, 3 ml of PBS + 2% FBS was added, and the cells were centrifuged again at 400g for 8 min and the supernatant was decanted. Cells were then pre-incubated with 5 µl of Human TruStain FcX (BioLegend, 422302) per 100 µl of cell suspension for 5 min at room temperature. The cells were then washed with PBS and centrifuged at 400g for 6 min. After decanting the supernatant, 10 µl of Brilliant buffer was added to each tube, and the mixture was allowed to sit for 5 min before addition of antibodies targeting extracellular markers and the cells were incubated for 20 min at room temperature in the dark. After incubation, cells were washed and fixed in the dark for 45 min at 4 °C with 500 µl of fixation solution (BD). Cells were then permeabilized with 2 ml permeabilization buffer. Intracellular stains were added in permeabilization buffer, and cells were incubated for approximately 40 min at room temperature. After incubation, cells were washed with permeabilization buffer and resuspended in 200 µl of PBS + 2% FBS. Analysis was then conducted using Cytek Aurora Spectral Flow Cytometer.
Preclinical experiments
All mouse experiments and procedures were approved by the University of Florida or the University of Texas MD Anderson Institutional Animal Care and Use Committee (IACUC). Mice (aged 4–10 weeks) were maintained at 21 ± 1 °C and 35% humidity under a 14 h–10 h light–dark cycle. Mice were randomized prior to treatment. Tumour measurements were performed blinded to the treatment group. Euthanasia was performed by CO2 inhalation followed by cervical dislocation in accordance with approved protocols. Humane end points included tumour ulceration.
C57BL/6 and Rigi-knockout mice (C57BL/6NJ-Rigiem1(IMPC)J/Mmjax) were purchased from The Jackson Laboratory (046070-JAX) and bred in house. Tumour-bearing mice were implanted s.c. with 50,000 B16F0 melanoma cells, 1 million B16F10-OVA melanoma cells or 200,000 LLC cells in the right flank. Orthotopic LLC models were implemented by implanting 100,000 LLC cells into the left lung of C57BL6 mice through direct injection below the 9th rib. A mix of male and female mice was used for experiments with B16F0, and male mice were used for experiments with LLC. All mice were vaccinated intramuscularly with 25 μg per dose of RNA-LNP or RNA-LPA (mRNA fraction)12. We administered anti-mouse IFNα receptor (aIFNAR1, Bio X Cell, BE0241) and anti-mouse IL-1R (Bio X Cell, BE0256) antibodies intraperitoneally at an initial dose of 500 μg per mouse for the first dose, followed by a maintenance dose of 250 μg per mouse twice a week for the remaining treatment period. Anti-PD-L1 (Bio X Cell, BE0101) checkpoint inhibitor was administered at 400 μg per mouse for the initial dose, followed by a maintenance dose of 200 μg per mouse twice a week for the remaining treatment period. Anti-PD-1 (Bio X Cell, BE0146) checkpoint inhibitor was administered at an initial dose of 400 μg per mouse, followed by a maintenance dose of 200 μg per mouse. LMW poly(I:C) (InvivoGen, tlrl-picw) was administered intramuscularly with 25 μg per mice for two doses. Tumours were measured at a frequency of three times a week starting on day 8 until more than 20% of mice reached the end point. Mice were euthanized after reaching the humane end point.
mRNA
SARS-CoV-2 spike coding sequence with K986P and V987P mutations was inserted into a pGEM-4Z backbone downstream of the T7 promoter with previously published UTRs and poly(A) signal analogous to the mRNA in BNT162b230,31. The 5′ SpeI restriction site was removed from the sequence to allow for restriction of the poly(A) tail. The T7 promoter was changed to have an AGG initiator sequence by site-directed mutagenesis (NEB, E0554) according to the company’s recommended protocol. Plasmids were grown in NEB5a competent Escherichia coli and purified using the RNeasy Maxi kit (Qiagen, 75162) and sequenced using whole-plasmid sequencing by Genewiz. Plasmids were restricted by using 2 U μg−1 SpeI HF (NEB, R3133L) for 2 h at 37 °C followed by DNA precipitation. mRNA was synthesized using the mMESSAGE mMACHINE T7 mRNA Kit with CleanCap Reagent AG (Thermo Fisher Scientific, A57620). Spike mRNA was made using CleanCap reagent AG (3′ OMe) (Trilink, N-7413-5) and N1-methylpseudouridine-5′-triphosphate (Trilink, N-1081-10). PP65 mRNA was capped using ARCA (NEB, S1411L) followed by treatment with mRNA cap 2′-O-methyltransferase (NEB, M0366L). The in vitro transcription reaction was performed at 20 °C for 10 h. DNA was removed according to the kit instructions and RNA was purified using the RNeasy maxi kit (Qiagen, 75162). RNA was eluted in purified RNase-free water and stored at −80°C until use. mRNA was checked for quality using an Agilent TapeStation 4150. After thawing, RNA was diluted to 100 µg ml−1 in RNase-free water followed by incubation at room temperature or 72 °C for 3 min. Then 100 ng of RNA or 1 µl of ladder was added to 5 µl of RNA ScreenTape sample buffer (Agilent, 5067-5577) and mixed at 2,000 rpm for 1 min. The samples were run using an RNA ScreenTape (Agilent, 5067-5576). For dsRNA removal, the method described previously was followed20. In brief, mRNA was precipitated and reconstituted in chromatography buffer. Cellulose fibres (Sigma-Aldrich, C6288-100G) suspended in chromatography buffer were added to Nucleospin filter units (Macherey-Nagel, 740606) followed by washing. Up to 500 μg of RNA per filter unit was added to cellulose and incubated by rapid mixing for 30 min. RNA was recovered by centrifugation, and incubation was repeated using a second column containing cellulose fibres. RNA was then filtered through a 0.45 µm syringe filter (Pall, 4604) to remove any cellulose particulate before being precipitated and reconstituted in RNase-free water20.
Fabrication of LNPs
Before complexation, 0.5 M of RNase-free citrate buffer (pH 3.75) (Teknova, Custom order) was added to the RNA for a final concentration of 0.1 M. ALC-0159 (Avanti, 880155 P), ALC-0315 (Avanti, 890900 O), cholesterol (Sigma-Aldrich, C8667) and DSPC (Avanti, 850365P) were reconstituted in 100% ethanol at a ratio of 1.7:47.5:40.8:10. Lipids and RNA were mixed at a 3:1 FRR on a Nanonsemblr Ignite (Precision NanoSystems, now part of Cytiva) for an N/P ratio of 6. RNA-LNPs were dialysed overnight at 4 °C with two buffer exchanges at the 3 h and 6 h timepoints using a 3.5 kDA dialysis cassette (Thermo Fisher Scientific, A52967). After dialysis, LNPs were filtered through the 0.2 µM Supor EX ECV filter (Pall, KS2ECV2S). LNPs were concentrated using 30 kDA Amicon filters (Millipore, UFC903024) by centrifugation at 2,000g. The final LNP formulation had sucrose dissolved in PBS added for a final concentration of 12%. Anionic RNA-LPAs were made as previously described12. In brief, DOTAP liposomes 2.5 mg ml−1 were mixed with mRNA at a 1:1 mass to mass ratio and complexed for 15 min at room temperature before administration.
RNA concentration was determined in LNPs using the RiboGreen assay (Invitrogen, R11490) using the BioTek Cytation 3 plate reader. The encapsulation efficiency was determined by comparing readings to LNPs in TE buffer versus LNPs in 1% Triton X-100. LNPs were diluted up to 500 μg ml−1 with PBS before injection. Empty LNPs were used at a volume consistent with the dose of RNA-LNPs fabricated using the same steps and same amounts of lipids. Fluorescence values were plotted in a standard curve with an extrapolation factor set to 1.1. The values from wells with Triton X-100 (total mRNA) and TE buffer (free mRNA) was entered into the following equation: EE% = [1 – (Free RNA/Total RNA)] × 100%. Total mRNA RNA-LNPs were also loaded onto a 1% agarose gel made in 1× TAE buffer (Quality Biological, 351-008-131) with GelRed (Biotium, 41003-T). Then 500 ng of RNA was loaded into LNPs and free RNA was mixed with 6× SDS-free (NEB, B7025S) loading dye and run at 90 V for 45 min. Particle size was acquired using a Malvern Zetasizer Ultra and NanoSight’s NS300. Before particle-size, PDI, concentration and surface ζ potential analysis, LNPs were diluted 100-fold with HyClone HyPure water (Cytiva, SH30538.02). They were and run in triplicate at 25 °C on the Zetasizer. Orthogonal particle-size distribution and concentration measurements were carried out using Malvern’s NanoSight NS300 NTA equipment. Each LNP sample was diluted 500-fold with PBS and pumped through the equipment at constant speed for five captures with optimized camera settings. An optimized detection threshold was set for analysis. mRNA was extracted from LNPs by dissolving 750 μg ml−1 with 5 volumes of ethanol or 2 volumes of isopropanol followed by 2 washes with cold 70% ethanol. RNA pellets were dissolved in RNase-free H2O before analysis using the TapeStation.
Plasma collection
Mice were bled through the retroorbital route using heparinized capillaries (Thermo Fisher Scientific, 22-362566) collecting a maximum of 200 µl into EDTA coated tubes (Thermo Fisher Scientific, NC9414041). Whole blood was centrifuged at 1,200g for 15 min. Plasma was collected and stored at −80 °C until use.
Processing tumours for flow cytometry analysis
Tumours were dissected from euthanized mice with the external fibrous sac left intact. Tumours were bisected using a scalpel and half of each sample was placed into a GentleMacs column GentleMACS C Tube (130-096-334) with an enzyme mix containing 10 mg ml−1 of collagenase (Sigma-Aldrich, C5138-1G), 1 mg ml−1 of hyaluronidase (StemCell Technologies, 07461) and 200 mg ml−1 of DNase (Sigma-Aldrich, D5025-150KU) or using the tumour dissociation kit (Miltenyi, 130-096-730) followed by debris removal (Miltenyi, 130-109-398). Samples were run on a m_TDK_1 cycle and centrifuged at 300g for 5 min after completion. The pellets were resuspended in cold PBS and filtered through a 70-µm cell strainer. The samples were washed twice using cold PBS and then manually counted using a haemocytometer.
Isolation of splenocytes
Whole spleens were collected from euthanized mice and placed into cold RPMI medium. The spleens were then teased through a 70-µm filter and lysed for 5 min at 37 °C using 1× BD Pharmalyse buffer (BD, 555899). Lysis buffer was quenched with medium and centrifuged at 500g for 5 min. Splenocytes were resuspended in cold PBS and filtered through a 70-µm cell strainer and washed once with PBS before being counted using the Beckman Coulter Vi-cell XR or haemocytometer.
Mouse flow cytometry analysis
In total, 1 × 106 cells from either tumours and spleens were placed into 96-well V-bottom plates. Unless stated otherwise, the washing steps were performed by centrifugation at 500g for 5 min at 4 °C followed by resuspension of cells with 200 µl of buffer, with mixing done by pipetting. Cells were washed with cold PBS and stained with 100 µl of live/dead stain (Thermo Fisher Scientific, L10119) for 30 min at 4 °C. Live/dead dye was quenched with 100 µl of cold PBS and the cells were washed once with cold FACS buffer (PBS with 2% FBS). Cells were centrifuged and resuspended with 10 µl True stain FCX buffer (BioLegend, 422302) diluted to 100 µg ml−1 with FACS buffer for 10 min. Then 90 µl of antibodies (Supplementary Tables 12 and 13) and Brilliant Stain buffer (BD, 563794) were added and incubated for 30 min at 4 °C. Next, 100 µl of FACS buffer was added to each well and the cells were washed twice with cold FACS buffer. Cells were fixed for 15 min with 100 µl of Cytofix buffer (BD, 554655) at 4 °C. Next, 100 µl of PBS was added to each sample and cells were washed twice and stored in FACS buffer at 4 °C in the dark until analysis. Initial compensation was acquired using Ultracomp eBeads (Thermo Fisher Scientific, 01-2222-42) and ArC amine reactive compensation beads (Thermo Fisher Scientific, A10346). Results were acquired using a BD Symphony A3 and analysed using FlowJo v.10.8.1 and v.10.10.0.
Tetramer production
Peptides selected to be high-affinity binders for Survivin (ATFKNWPFL), GP100A (EGSRNQDWL), GP100B (KVPRNQDWL), WT1 (RMFPNAPYL), CLDN6 or claudin 6 (KVYDSLLAL) and TRP2 (SVYDFFVWL) were purchased from GenScript. H2-Db and H2-Kb monomers were purchased as easYmers from ImmunAware (5001-01 and 5004-01, respectively), and tetramers were prepared according to the manufacturer’s instructions. In brief, peptides were reconstituted to 1 mg ml−1 in deionized water, further diluted to 75 μM and incubated with easYmer (Eagle Biosciences, 5004-01, 5001-01) at 18 °C for 48 h. The resulting complexes were tetramerized by gradually mixing with streptavidin-APC (BD Biosciences, 554067). After incubation, a final stock concentration of 500 nM for each tetramer was achieved.
Tetramer staining
According to the manufacturer recommendations, 1–2 × 106 cells were stained with tetramer diluted to 20 nM in FACS buffer. Cells were left to stain in the dark at room temperature for 1 h, washed once with FACS buffer, co-stained with surface antibodies (CD3, CD4) at room temperature for 20 min, washed twice with FACS buffer and analysed on a Cytek Aurora flow cytometer.
AIM assay
The AIM assay was performed as previously described12. In this iteration, whole splenocytes were used rather than isolated T cells. In brief, 24 h after the last RNA-LNP vaccine, splenocytes were collected for antigen recall assay. Spleens were collected and processed as described above. 1 million splenocytes were cultured in a 96-well round-bottom plate in T cell medium containing RPMI 1640 (Gibco, 11-875-119), 10% FBS (Thermo Fisher Scientific, 35-011-CV), 1% penicillin–streptomycin (Gibco, 30-002-CI), 1% MEM non-essential amino acids (NEAA, Gibco, 11140050), 1% sodium pyruvate (Gibco, 11360070), 0.1% β-mercaptoethanol (BME, Gibco, 21985-023) and 0.2 µg tumour-associated peptides without cytokines. Peptides were chosen based on their suggested upregulation in melanoma tumours12. Selected peptides were purchased from JPT Peptide Technologies and reconstituted according to the manufacturer’s guidelines. Co-culture was maintained for 48 h in an incubator at 37 °C under 5% CO2. Cells were next collected and stained for the AIM assay for evaluating activation of antigen-specific T cells. Cells were centrifuged and resuspended in FACS buffer containing antibodies mix (Supplementary Table 14). Cells were washed three times and fixed using fixation medium (Thermo Fisher Scientific, GAS001S100) for 15 min at room temperature. Cells were washed once with PBS and were stored in FACS buffer at 4 °C in the dark until analysis. Results were acquired using a BD Fortessa III. Values were normalized by subtracting the percentage of AIM+ cells of tbe DMSO-only controls from each treatment condition. Statistical analysis was performed using Brown–Forsythe and Welch ANOVA, followed by Dunnett’s T3 multiple-comparison test.
ELISA and multiplex analysis
Plasma was analysed for cytokine concentration using ELISAs and multiplex cytokine arrays. A portion of the plasma samples were assessed for IFNα through ELISA (Invitrogen, BMS6027). Plasma was also sent for analysis by Eve Technologies for multiplex cytokine array (MD44). For quantification of spike IgG, mice that received three vaccines/vehicle doses during tumour studies were bled once the humane end point was reached. Spike-specific IgG was determined using an Anti-mouse SARS-CoV-2 IgG titre assay (AcroBiosystems, RAS-T023). Plasma was diluted 100,000-fold and run according to the company’s recommended parameters for semiquantitative analysis.
Immunofluorescence
Half of bisected tumours were fixed with 4% paraformaldehyde at 4 °C overnight. The samples were washed three times using PBS and then immersed successively into 10%, 20% and 30% sucrose for cryopreservation. Tissue was then embedded in O.C.T. (Tissue-Tek, 4583) and stored at −80 °C. Blocks of tissue were moved to −20 °C for 24 h and were sectioned at a thickness of 30 µm using the Leica cryostat, and the sections were placed onto microscope slides. Before staining, the slides were brought up to room temperature for 15 min and washed three times with PBS. Tissue was blocked at room temperature for 1 h using a blocking buffer containing 2% goat or donkey serum, 1% BSA and 0.1% Triton X-100 in PBS. The sections were then stained with primary antibodies (Supplementary Table 15) in blocking buffer overnight at 4 °C. After incubation, the sections were washed three times with PBS and stained with secondary antibodies (Supplementary Table 15) diluted in blocking buffer for 1 h at room temperature. After three 5 min PBS washes, the sections were incubated with DAPI (1:1,000 in PBS) for 10 min at room temperature. After a triple PBS wash, the sections were mounted with Prolong Glass Antifade Mountant (Thermo Fisher Scientific, P36984) and covered with a cover glass. Images were acquired using a Leica Stellaris 8 WLL Spectral Confocal Microscope. Image processing was performed using Fiji ImageJ software (NIH) and Imaris (Oxford Instruments).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.