Orthologue identification, phylogenetic analysis and sequence alignment
We used previously identified Cas12a2 protein sequences27 as queries for tBLASTn and BLASTp searches in the NCBI databases (https://www.ncbi.nlm.nih.gov) and the JGI Integrated Microbial Genomes and Microbiomes database (https://img.jgi.doe.gov) to identify closely related orthologues. The resulting amino acid sequences, along with Cas12a orthologues used as an outgroup, were aligned using Clustal Omega59. The trimmed alignment generated using ClipKIT60 was then used to reconstruct a phylogeny using IQ-TREE (v.2.3.6) (-m MFP -T 8 -B 1000)60,61 with a maximum-likelihood approach. Assignment of putative domains and conserved amino acid residues, as shown in Fig. 1a,b and Extended Data Fig. 1, was performed with reference to SuCas12a2 (refs. 27,28). The amino acid sequences of the nucleases are provided in Supplementary Data 1. Information on each nuclease, including contig accession numbers, source organisms and the presence of spacer acquisition genes (cas1, cas2 and cas4), is provided in Supplementary Table 1. The presence of these genes was determined using DefenseFinder (v.2.0.1) with defense-finder-models (v.2.0.2)62. CRISPR arrays were identified using CRISPRCasFinder (v.4.2.21)63.
Strains, plasmids and oligonucleotides
Strains, plasmids and oligonucleotides used in this study are listed in Supplementary Table 2. Nuclease-encoding sequences were codon-optimized for expression in E. coli and synthesized by Twist Bioscience, unless stated otherwise. DNA oligonucleotides and FAM-labelled reporters were synthesized by Integrated DNA Technologies.
PFS screen in E. coli
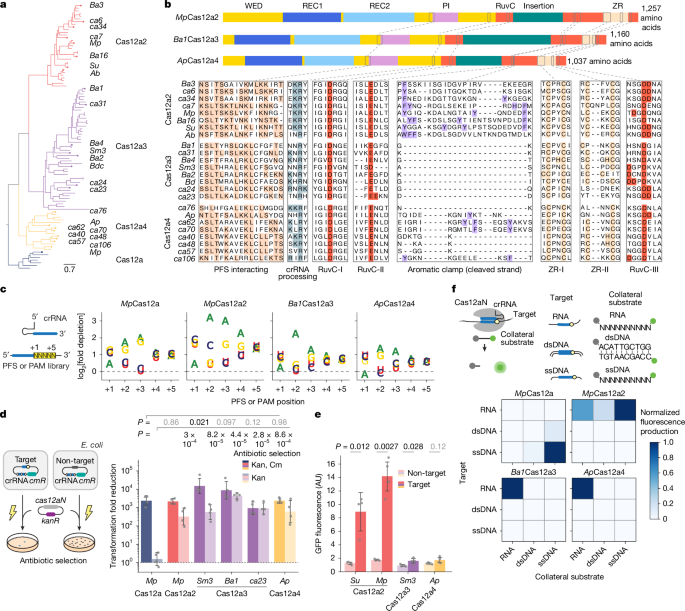
A PFS-containing plasmid library (CBS-6873) was constructed by incorporating a target sequence (CAO1: 5′-CAUCAAGCCUUCCUUCAGGUGUUGCUCCA-3′) followed by 1,024 combinations of five randomized nucleotides (NNNNN). Thus, the target, placed under the PJ23119 promoter (https://parts.igem.org/Promoters/Catalog/Anderson) was cloned into a low-copy sc101 plasmid (around 5 copies per cell), which was then amplified using the primers ODpr23 and ODpr24 (Supplementary Table 2), with the forward primer including a 5-nucleotide randomized overhang. After DpnI treatment to remove template DNA, the resulting PCR product was ligated and electroporated into E. coli TOP10, which produced >2 million transformants (about 2,000-fold library coverage). The PFS preferences of Cas12a, Cas12a2, Cas12a3 and Cas12a4 orthologues was assessed by targeting the CBS-6873 library with a CAO1-targeting crRNA plasmid (CBS-6875), using a non-targeting crRNA plasmid (CBS-6876) as a control. The nucleotide-encoding sequences were codon-optimized for E. coli and expressed under a T7 promoter, whereas crRNAs were driven by the PJ23119 promoter. E. coli BL21(AI) cells with the nuclease and crRNA plasmids were electroporated in three separate reactions, each using around 500 ng of library DNA in 50 µl competent cells recovered in LB with 0.1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and 0.2% l-arabinose and grown overnight to produce about 2 million transformants (>2,000-fold library coverage). Plasmids were then purified using a ZymoPURE II Plasmid Midiprep kit (D4201). Reactions for each experimental condition were carried out in duplicate.
Purified plasmids from both target and non-target conditions were first PCR-amplified using the primers ODpr55 and ODpr56 (Supplementary Table 2) with KAPA HIFI HotStart polymerase (KK2601) for 20 cycles at 64.5 °C following the manufacturer’s protocol. After amplification, these PCR products were purified using AMPureXP beads (Beckman Coulter, A63880) and subsequently indexed for Illumina sequencing using standard indexing primers with KAPA HIFI HotStart polymerase (KK2601) for 8 cycles at 61.5 °C with 2 µM forward and reverse primers and 5 ng µl–1 DNA. The resulting indexed PCR products were sequenced on an Illumina NovaSeq 6000 (paired-end, 150 bp reads), which ensured that at least 2 million reads per sample were sequenced. Raw FASTQ files were processed with Trimmomatic (v.0.39)64 using the following parameters: ILLUMINACLIP:TruSeq3-PE.fa:2:30:10, LEADING:3, TRAILING:3, and SLIDINGWINDOW:4:15. Paired-end reads were merged using BBMerge (qtrim=t, trimq=10, minlength=20)64,65. Sequences containing motifs matching “TTCCTTCAGGTGTTGCTCCA (…..) GGTGAGTTCT”, corresponding to the 20-nucleotide target-encoding sequence and the 10-nucleotide downstream sequence, were extracted, excluding any sequences with ambiguous bases (N) or Phred scores below 20. Depletion scores were then calculated using the formula: depletion = (sum(non-target)/sum(target)) × (count(target)/count(non-target)). The log2 fold change values for these scores were computed for the nucleotides at PFS positions (+1 to +5), and scatterplots visualizing the PFS preferences were generated using Matplotlib in Python.
Plasmid clearance in E. coli
To test plasmid clearance, we expressed E. coli codon-optimized orthologues (as in the PFS screen) from plasmids (Supplementary Table 2). Target RNA and crRNA were co-expressed from a single plasmid under separate PJ23119 promoters (plasmids CBS-6177–6182 in Supplementary Table 2 include CAO1, GAPDH and GFP targets with both target and non-target crRNAs). E. coli BL21 (AI) cells with these target–guide constructs were electroporated with 1 µl of high-purity 50 ng µl–1 nuclease plasmid. Cells were then recovered in LB with 0.2% l-arabinose and 1 mM IPTG at 37 °C for 1 h with shaking without antibiotics. Serial tenfold dilutions (up to 10–1 in 1× PBS) were then prepared, and 5 µl of each dilution was spotted onto LB agar plates containing 0.2% l-arabinose, 0.1 mM IPTG and the appropriate antibiotics, including 50 µg ml–1 kanamycin for selection of the nuclease plasmid alone (assessing growth arrest) or 50 µg ml–1 kanamycin plus 25 µg ml–1 chloramphenicol for co-selection (assessing plasmid clearance). Plates were incubated overnight at 37 °C. Experiments were performed in four biological replicates.
relA deletion in E. coli
Deletion of the relA gene in E. coli BL21(AI) was performed using λ Red recombineering as previously described66. To initiate recombineering, wild-type E. coli BL21(AI) cells were transformed with plasmid pKD46 (Supplementary Table 2), recovered and incubated overnight at 28 °C on LB agar containing ampicillin. The kanamycin resistance (kanR) cassette was PCR-amplified from plasmid pKD13 using the primers ODpr1652 and ODpr1653, which included 50 bp homology arms targeting sequences immediately upstream and downstream of the relA locus (positions 2,715,617–2,717,851 on GenBank accession CP047231), which encodes GTP pyrophosphokinase (NP_311671.1). The resulting PCR product was electroporated into E. coli cells with pKD46, which had been induced with 0.2% l-arabinose to express the λ-RED recombination machinery. Cells were plated on LB agar containing kanamycin and incubated at 37 °C to select for successful recombinants. To remove the inserted kanR cassette, plasmid pCP20 (Supplementary Table 2), which encodes FLP recombinase, was transformed into the strain. The cassette was excised via recombination at the flanking FRT sites, and pCP20 was subsequently cured by incubation at 37 °C. Successful deletion of relA was verified by colony PCR using the primers ODpr1655 and ODpr1654 (Extended Data Fig. 4), and by confirming the absence of growth on kanamycin-containing plates. Four independent mutants were generated and used to perform plasmid clearance (Extended Data Fig. 4), with the GAPDH T17 target under targeting and non-targeting conditions, using the plasmids CBS-6179 and CBS-6180 (Supplementary Table 2), respectively.
SOS response induction in E. coli
To quantify the recA-dependent SOS response, E. coli BL21 (AI) cells were co-transformed with the carbenicillin-resistant reporter PrecA-GFP (CBS-3611) or a noGFP control (CBS-3616) along with the nuclease and target–guide plasmids, performed as previously described27. The resulting overnight cultures were grown in LB containing 100 µg ml–1 carbenicillin, 50 µg ml–1 kanamycin, 25 µg ml–1 chloramphenicol and 0.2% glucose to repress nuclease expression. The next day, 1 ml of each culture was pelleted (5,000g, 2 min), resuspended in fresh LB without antibiotics and adjusted to an OD600 of 0.1. Then, 20 µl of each culture was added to 180 µl fresh medium to obtain a final concentration of 0.2% l-arabinose and 0.1 mM IPTG in a 96-well plate (final OD600 = 0.01). Samples were incubated at 37 °C with vigorous shaking in a BioTek Synergy H1 plate reader, with OD600 and GFP fluorescence (excitation of 485/20 nm, emission of 528/20 nm) recorded. Each condition was tested in four biological replicates.
Protection against phage T4 infection
Fresh E. coli BL21 (AI) cells at OD600 of 0.3 containing the respective nuclease (CBS-7169 and CBS-7171) and guide (CBS-7181-7183) plasmids (Supplementary Table 2) were grown in LB medium supplemented with 50 µg ml–1 kanamycin, 25 µg ml–1 chloramphenicol, 0.2% l-arabinose and 0.1 mM IPTG to induce nuclease expression. These cultures were challenged with different multiplicities of infection of T4 phage and incubated at 37 °C with vigorous shaking overnight. The next day, the cultures were serially diluted in tenfold increments in SM buffer (50 mM Tris-HCl, 100 mM NaCl and 8 mM MgSO4, pH 7.5). For plaque assays, control E. coli BL21 (AI) cells with an empty carbenicillin-resistant plasmid were mixed with 0.75% top agar and poured onto LB agar plates containing 100 µg ml–1 carbenicillin. Serial dilutions of the overnight cultures were then spotted onto the top agar, and the plates were incubated overnight at 37 °C to allow plaque formation. Each experimental condition was performed in three biological replicates. GFP-targeting crRNA-expression plasmid (CBS-7184) was used as a non-target control (Supplementary Table 2).
In vitro determination of activating targets and cleavage substrates
RNAs were in vitro-transcribed from linear dsDNA templates containing a T7 promoter (Supplementary Table 2). Templates for the GAPDH T17 and T19 crRNAs were generated by PCR amplification of ssDNA oligonucleotides using the primers ODpr1220 and ODpr1221, and ODpr1220 and ODpr1223, respectively. The T17 target RNA template was amplified from plasmid CBS-7159 using the primers ODpr1212 and ODpr1213. In vitro transcription was performed using a HiScribe T7 High Yield RNA Synthesis kit (NEB, E2040) following the manufacturer’s instructions. The generated target RNA along with the complementary crRNA were also used for cryo-EM.
For the in vitro experiment, recombinant nucleases were purified primarily as previously described27,28. In brief, the proteins were expressed in E. coli BL21(DE3) star grown in LB medium and induced at an OD600 of about 0.6 with 0.1 mM IPTG, followed by an overnight incubation at 18 °C. Cells were then collected, lysed by sonication in lysis buffer and clarified by centrifugation at 30,000g for 30 min at 4 °C. The soluble fraction was then incubated with Ni-NTA resin (pre-equilibrated in lysis buffer) for 30 min at 6–8 °C, and the column was washed with an IMAC washing buffer supplemented with 2 M NaCl before bound proteins were eluted with imidazole-containing buffer. The eluate was diluted with low-salt buffer and further purified by ion-exchange chromatography on a HiTrap Heparin column using a NaCl gradient. Pooled fractions were concentrated and polished by size-exclusion chromatography on a HiLoad Superdex 200 column. The final purified fractions were pooled, concentrated, flash-frozen in liquid nitrogen and stored at −80 °C.
Preference for targets and cleavage substrates was evaluated in vitro using nucleases purified as described above. Reactions contained 500 nM of the respective nuclease, an equimolar amount of T19 GAPDH non-target crRNA and T17 GAPDH target crRNA, and T17 GAPDH target at 50 nM provided either as RNA, ssDNA or dsDNA, as well as respective non-target substrates at 1 µM (Supplementary Table 2). For assays with Ba1Cas12a3 Y922A and ΔtRLD (Supplementary Fig. 13), reactions contained 100 nM nuclease, crRNA and target RNA together with 5 µM RNA substrate library. Reactions were performed in a buffer comprising 40 mM Tris-HCl (pH 7.5), 50 mM NaCl and 1 mM DTT, with MgCl2 at 2 mM for Cas12a2, Cas12a3 and Cas12a4 nucleases and 10 mM for Cas12a. Fluorescence, indicative of reporter cleavage via separation of the fluorophore and quencher, was monitored at 29 °C on a BioTek Synergy H1 plate reader (excitation of 492 nm, emission of 520 nm). For the heatmap in Fig. 1f, initial reaction rates in the linear range were normalized for each nuclease by setting the highest rate across reporters to one. All experiments were performed in at least three biological replicates.
Nuclease activity assay in TXTL reactions
Activity of the purified Ba1Cas12a3 and MpCas12a2 nucleases was assessed using the TXTL system (Arbor Biosciences, 540300). Ba1Cas12a3 (25 nM) and MpCas12a2 (50 nM) were individually pre-incubated with 37.5 nM and 75 nM crRNA, respectively, for 15 min at 29 °C in 40 mM Tris-HCl (pH 7.5), 50 mM NaCl and 1 mM DTT, with 2 mM MgCl2. After this pre-incubation step, 50 nM GAPDH T17 RNA target was added and the mixtures were further incubated for 15 min at 29 °C to allow the formation of ribonucleoprotein particle (RNP) complexes. The assembled RNPs were then added to the TXTL reaction and incubated for 4 h at 29 °C. Thereafter, the deGFP plasmid supplied with the TXTL kit was added at 0.5 nM. In parallel, the RNP and the deGFP plasmid were simultaneously introduced into the TXTL mix that had already been pre-incubated for 4 h at 29 °C. GFP signal production was subsequently monitored on a BioTek Synergy H1 plate reader set to an excitation of 485 nm and an emission of 528 nm. All experiments were performed in at least three biological replicates.
RT–qPCR analysis
TXTL reactions were carried out using myTXTL (Arbor Biosciences, 540300) supplemented with the pCBS420 plasmid constitutively expressing deGFP and the pCBS11 p70-T7RNAP plasmid (Supplementary Table 2). For LshCas13a, the non-targeting plasmid CBS-3620 and the gfp targeting plasmid CBS-3622 expressing both the nuclease and crRNA were used; for Ba1Cas12a3, the non-targeting plasmid CBS-7245 and the targeting plasmid CBS-7246 were used (Supplementary Table 2). Final concentrations were 1 nM nuclease and crRNA co-encoded plasmids, 0.5 nM pCBS420, 0.33 nM p70-T7RNAP and 0.66 mM IPTG. Fluorescence was monitored over 16 h on a Synergy H1 plate reader (BioTek) at 485 nm excitation and 528 nm emission. For the monitoring of fluorescence, 3 µl volume of TXTL was used, whereas for RNA extraction, the reactions were scaled to 30 µl and sampled after 4 h, when strong GFP reduction under targeting conditions was evident. All experiments were performed in three biological replicates.
Following TXTL, reactions were treated with 10 µl proteinase K for 15 min at room temperature and RNA was purified using a RNA Clean & Concentrator-25 kit (Zymo Research, R1017) with on-column DNase I digestion. RT–qPCR was performed using an iTaq Universal SYBR Green One-Step kit (Bio-Rad, 172-5150) on a CFX Opus 384 Real-Time PCR system (Bio-Rad) with CFX Maestro (v.5.3.022.1030). Primer pair 1 (ODpr1662 and ODpr1663) and primer pair 2 (ODpr1670 and ODpr1671) were validated using a dilution series of RNA from TXTL expressing gfp from plasmid pCBS420 (Supplementary Table 2), which produced efficiencies of 94.7% and 97.4%, respectively. Each primer was used at 300 nM. Cycling conditions included a 63 °C annealing–extension step, with other parameters following the manufacturer’s protocol. Melting curve analysis confirmed single amplicons for both primer pairs. No amplification was detected in no-RT controls after 30 cycles. Each condition was tested in three biological replicates, with three technical replicates per sample.
Nanopore direct RNA sequencing and analysis
Ba1Cas12a3 and Sm3Cas12a3 nucleases (250 nM each) were pre-incubated in 40 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM DTT and 2 mM MgCl2 for 15 min at 29 °C with equimolar amounts of GAPDH T19 non-target crRNA and GAPDH T17 target crRNA. Next, GAPDH T17 target RNA was added to a final concentration of 250 nM and the mixture was incubated for at least 15 min to form the RNP complex. The assembled RNP was then added to 30 µl TXTL reactions. After 4 h of incubation at 29 °C, 6 μl of proteinase K (NEB, P8107) was added and the reactions were incubated for 15 min. RNA was purified from the reactions using a RNA Clean & Concentrator-25 kit (R1017, Zymo Research). To remove full-length rRNAs, RNAs with lengths of ≤200 nucleotides were isolated with a miRNeasy Tissue/Cells Advanced Micro kit (217684, Qiagen) according to the manufacturer’s instructions. Subsequently, tRNAs in the purified RNA were deacylated by incubation in 90 mM Tris-HCl (pH 9.0) at 37 °C for 30 min. The RNA was next recovered using a RNA Clean & Concentrator-25 kit with 1.3× ethanol concentration after the RNA prep buffer step. All reactions were performed in triplicate.
For the in vitro total E. coli tRNA cleavage reactions, Ba1Cas12a3, Sm3Cas12a3 and ca23Cas12a3 nucleases (750 nM each) were combined in 40 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM DTT and 2 mM MgCl2 buffer with an equimolar amount of crRNAs and incubated for 15 min at 37 °C. Next, an equimolar amount of target RNA was added and the mixture was incubated for an additional 15 min to allow RNP complex formation. Total tRNAs purified from E. coli MRE600 (10109541001, Roche) were then added to the final concentration of 1.5 µM. The reactions were subsequently incubated at 37 °C for 2 h followed by proteinase K treatment and purification using a RNA Clean & Concentrator-5 kit (R1013, Zymo Research). Finally, the recovered RNAs were deacylated as described for the TXTL samples. All reactions were performed in triplicate.
For each sample, 10 µg of total RNA was polyadenylated using E. coli poly(A) polymerase (NEB) in a 20 µl reaction containing 50 mM Tris-HCl pH 8, 250 mM NaCl, 10 mM MgCl2, 1 U µl–1 RNasin, 1 mM ATP and 0.25 U µl–1 poly(A) polymerase for 30 min at 37 °C. Polyadenylated RNAs were purified using a RNA Clean & Concentrator-5 kit (Zymo Research) following the manufacturer’s instructions. Multiplexed Nanopore direct RNA sequencing was then performed following a previously described strategy67. In brief, custom barcode containing RTA adapters (bc03, bc04 and bc011; Supplementary Table 2) were annealed at a final concentration of 10 µM in 30 mM HEPES-KOH (pH 7.5) and 100 mM potassium acetate by incubation for 1 min at 95 °C followed by a slow cooling to 25 °C at a rate of 0.5 °C min–1. The annealed RTA adapters were then diluted to 0.7 µM and stored at −20 °C until use. Next, 100 ng of polyadenylated RNA was ligated to 1 µl of 0.7 µM annealed RTA in the presence of 2 µl of 5× NEBNext Quick ligation buffer (NEB), 0.5 µl of high-concentration T4 DNA Ligase (M0202M, NEB) and 0.2 µl of RNAsin (Promega) in a final volume of 10 µl by incubation at 22 °C for 15 min. To stop the ligation process, 2 µl of 0.5 M EDTA was added and samples were pooled and purified with 0.5 volumes of SPRI beads (Mag-Bind TotalPure NGS, Omega Bio-tek), washed twice with 200 µl fresh 80% ethanol and eluted in 10 µl RNase-free H2O. RT was performed by adding 8 µl of 5× Induro buffer, 2 µl of 10 mM dNTP, 2 µl of 10 µM random hexamer oligonucleotides, 2 µl Induro RT (NEB), 0.5 µl RNasin in a final volume of 40 µl and incubation 2 min at 22 °C (annealing), 15 min at 60 °C (RT) and 10 min at 70 °C (heat-inactivation). The reaction was purified with 1.4 volumes of SPRI beads, washed twice with 200 µl of fresh 80% ethanol and eluted in 10 µl RNase-free water. Sequencing adapter ligation was performed using a SQK-RNA004 library kit (ONT) according to the manufacturer’s instructions. In brief, 10 µl of purified library was mixed with 6 µl 5× NEBNext Quick ligation buffer, 5 µl of RLA sequencing adapter (ONT SQK-RNA004) and 3 µl of T4 DNA Ligase high concentration in a final volume of 30 µl and incubated 15 min at 22 °C. The reaction was purified with 0.5 volumes of SPRI beads and washed twice with 100 µl RNA wash buffer (WSB, ONT SQK-RNA004). The library was then eluted in 32 µl RNA elution buffer (REB, ONT SQK-RNA004). For sequencing, the libraries were loaded onto a PromethION (FLO-PRO004RA) flow cell for RNA samples originating from the TXTL reactions or onto a MinION (FLO-MIN004RA) flow cell for RNA samples originating from the in vitro reactions. Data acquisition was performed using MinKNOW (v.24.11). Basecalling was performed with dorado (v.0.8.3) using the sup model. Barcode classification was performed using the demux command of warpdemux (v.0.4.4) with the WDX12_rna004_v0_4_4 model67. Unaligned bam files were then demultiplexed based on barcode labels using calibrated target performance scores.
Non-coding RNA sequence references for mapping the Nanopore reader were identified in the E. coli BL21(DE3) genome (CP010816) for TXTL analysis and the E. coli MRE600 genome (CP014197) for the analysis of the in vitro tRNA cleavage experiments. In silico identification were performed using covariance models from Rfam68 for tRNA (RF00005), tmRNA (RF00023), 5S rRNA (RF00001), 16S rRNA (RF00177) and 23S rRNA (RF02541) with searches executed using cmsearch69. The tRNA hits were subsequently annotated using tRNAscan-SE (v.2.0)70 to generate the final set of tRNA sequences for analysis. The resulting reference fasta files are provided in Supplementary Data 2 and 3.
The poly(A) tail sequences were trimmed and the reads subsequently mapped to the reference using BWA-MEM (v.0.7.17)71 with the minimum seed length of 19 (-k 19) and the minimum alignments score of 30 (-T 30). The -M option was enabled to mark shorter split hits as secondary alignments. The resulting SAM files were converted to BAM files, sorted and indexed using SAMtools (v.1.9)72. Reads with a mapping quality (MAPQ) below 20 were filtered out before downstream analyses.
Depletion scores were computed for each nucleotide position according to the formula: depletion = (sum(non-target)/sum(target)) × (count(target)/count(non-target)). In this equation, ‘sum’ denotes the total number of reads mapped to a reference sequence (that is, the overall read count) under either non-target or target condition, whereas ‘count’ refers to the per–nucleotide coverage (the number of reads overlapping that specific position). This normalization procedure produces a relative measure of depletion at each nucleotide position.
The z scores for each nucleotide position were calculated using the per-nucleotide depletion score, the overall mean and standard deviation for each sequence reference according to the formula: z = (nucleotide depletion score – reference mean)/reference standard deviation. For visualization purposes, the z scores were then constrained to a range of 0 to 2.
Fluorescence in vitro tRNA cleavage
Total purified E. coli tRNAs (Roche, 10109541001) were labelled at the 5′ end with Cy5 using a Vector Labs kit (MB-9001) according to the manufacturer’s protocol, with Cy5 maleimide monoreactive dye (GEPA15131, Sigma-Aldrich). For 3′ end labelling, total E. coli tRNAs and Saccharomyces cerevisiae tRNA (Life Technologies, 0000010468) were first deacylated in 90 mM Tris-HCl (pH 9.0) at 37 °C for 30 min. Up to 400 pmol of RNA was then combined with 1 mM ATP, 100 µM pCp-Cy5 (NU-1706-CY5, Jena Bioscience), 1 µl T4 RNA ligase (M0202, NEB) and 1 µl of 100% DMSO in the reaction buffer supplied with the ligase. The reaction was incubated overnight at 16 °C. In vitro-transcribed tRNAs were labelled at the 3′ end using the same protocol.
To generate in vitro-transcribed tRNAs, DNA templates were first prepared by PCR using the following primer pairs: ODpr1343 and ODpr1344 for tRNALys(UUU); ODpr1345 and ODpr1346 for tRNAAla(UGC); ODpr1347 and ODpr1348 for tRNASer(GGA); and ODpr1349 and ODpr1350 for tRNATyr(GUA). In vitro transcription was then carried out using a HiScribe T7 High Yield RNA Synthesis kit (NEB, E2040) according to the manufacturer’s instructions.
To generate truncating tRNAAla(UGC), self-annealing PCR reactions were performed using the following primer pairs: ODpr1711 and ODpr1712 for ΔA; ODpr1715 and ODpr1716 for ΔT; ODpr1713 and ODpr17134 for ΔD; ODpr1717 and ODpr1718 for ΔTD; ODpr1719 and ODpr1720 for ΔAD; and ODpr1739 and ODpr1740 for h1 (Supplementary Table 2). In vitro transcription was performed using a HiScribe T7 High Yield RNA Synthesis kit (NEB, E2040) according to the manufacturer’s instructions, using up to 1 µg of PCR template.
For the in vitro digestion assay, the nucleases MpCas12a, Ba1Cas12a3, Sm3Cas12a3, ca23Cas12a3 and ApCas12a4 were each used at 250 nM, combined with either equimolar GAPDH T19 non-target or T17 target crRNAs. To activate the proteins, 10 nM target RNA was added, followed by the addition of Cy5-labelled tRNA substrates at 50 nM. The reactions were performed in 40 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM DTT and 2 mM MgCl2 and incubated for 2 h at 37 °C. Reaction products were subsequently analysed by electrophoresis on a 10% polyacrylamide gel containing 7 M urea. All reactions were performed in triplicate.
Northern blotting
For northern blot analysis, TXTL and the in vitro tRNA cleavage reactions were performed as for Nanopore sequencing. After purification, 10 μg of each RNA sample from the TXTL reactions and 1 µg from the in vitro total purified E. coli tRNA reactions were resolved on an 8% polyacrylamide gel containing 7 M urea at 300 V for 2 h and 25 min using a gel transfer system (Doppel-Gelsystem Twin L, PerfectBlue). Using an electroblotter with an applied voltage of 50 V for 1 h at 4 °C (Tank-Elektroblotter Web M, PerfectBlue), the RNA was transferred onto Hybond-XL membranes (Sigma-Aldrich, 15356-1EA), crosslinked with UV-light for a total of 0.12 Joules (UV-lamp T8C; 254 nm, 8 W) and hybridized overnight in 15 ml of Roti-Hybri-Quick buffer at 42 °C with 5 μl γ-[32P]-ATP end-labelled oligodeoxyribonucleotides (5 pmol μl–1). Probe ODp7 was used for tRNAAla(UGC), probe ODp17 for tRNALeu(UAG) and probe ODp6 for 5S rRNA (Supplementary Table 2). The labelled RNA was visualized with a Phosphorimager (Typhoon FLA 7000, GE Healthcare).
Fluorophore–quencher in vitro cleavage
LwaCas13a protein was obtained from GenCRISPR (Z03486), whereas purified PsmCas13b was provided by M. Kaminski’s Laboratory. Target RNA and crRNAs for Cas12a3 nucleases were prepared as described above. For PsmCas13b, PCR amplification was performed using the complementary primers ODpr1448–1449 to generate the GAPDH T17 target crRNA template, and the primers ODpr1450–1451 for the non-target (T19) crRNA template. Similarly, for LwaCas13a, the corresponding crRNA templates were generated by PCR using the primers ODpr1424–1425 for the target and the primers ODpr1426–1427 for the non-target crRNAs. The respective RNAs were in vitro-transcribed using a HiScribe T7 High Yield RNA Synthesis kit (NEB, E2040).
Reactions were performed in 40 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM DTT and 2 mM MgCl2 using nuclease-specific reporters as listed in Supplementary Table 2. For tests with tRNA-mimicking substrates using Cas12a3 nucleases, Ba1Cas12a3 (125 nM), Sm3Cas12a3 (500 nM) and ca23Cas12a3 (500 nM) were each combined with 500 nM of either target or non-target crRNA, 50 nM of GAPDH T17 RNA target and 1 mM of fluorescent RNA reporters. In experiments assessing orthogonality, Ba1Cas12a3 (100 nM), LwaCas13a (10 nM) and PsmCas13b (250 nM) were used with an equimolar amount of target or non-target crRNA, with target RNA added at 50 nM and fluorescent RNA reporters at 1 mM. Reactions were carried out at 29 °C in BioTek Synergy H1 plate reader with fluorescence monitored at an excitation of 492 nm and emission of 520 nm. Fluorescence production rates were determined in the initial linear range, normalized for each nuclease, and analysed as a function of nuclease concentration. All experiments were performed at least in triplicate.
For catalytic efficiency calculations with substrates S13, S14 and h25, reactions contained 50 nM Ba1Cas12a3, crRNA and target RNA, together with fluorophore–quencher reporters at 5,000, 2,500, 1,250, 625 or 312.5 nM. Reactions were performed in a buffer containing 40 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM DTT and 2 mM MgCl2. Fluorescence was monitored over time, and rates of fluorescence increase were background-corrected using the corresponding non-target controls. Rates were fitted to the Michaelis–Menten equation to extract kinetic parameters, and catalytic efficiency was determined as kcat Km–1. Each condition was tested in at least three independent replicates.
To compare truncated tRNA substrates with mutations in the 3′ CCA tail, reactions contained 100 nM Ba1Cas12a3, crRNA and target RNA together with 1 µM reporters h25–h29 in buffer (40 mM Tris-HCl pH 7.5, 50 mM NaCl, 1 mM DTT and 2 mM MgCl2). Fluorescence was monitored over time, with each condition tested in at least three independent replicates.
DNA templates for viral RNA targets were generated by PCR using the primers ODpr1684 and ODpr1685 for SARS-CoV-2 (N gene), ODpr1686 and ODpr1687 for influenza A (NP gene), and ODpr1690 and ODpr1691 for RSV (N gene) (Supplementary Table 2). crRNA templates were generated by PCR as follows: Ba1Cas12a3 non-targeting control (ODpr1692 and ODpr1693); Ba1Cas12a3 RSV N target (ODpr1692 and ODpr1738); LwaCas13a non-target (ODpr1424 and ODpr1696); LwaCas13a influenza A NP target (ODpr1424 and ODpr1697); PsmCas13b non-target (ODpr1701 and ODpr1702); and PsmCas13b SARS-CoV-2 N target (ODpr1709 and ODpr1710) (Supplementary Table 2). All RNAs were transcribed in vitro using a HiScribe T7 High Yield RNA Synthesis kit (NEB, E2040).
Purified Ba1Cas12a3, LwaCas13a and PsmCas13b were each assembled with their cognate crRNAs at 100 nM and tested with target RNA ranging from 50 nM to 0.5 nM. Universal Human Reference RNA (Life Technologies, QS0639) was added at 10 or 50 nM, calculated assuming an average RNA length of 2 kb. Reporters were S11 (TEX fluorophore) for LwaCas13a, S12 (HEX) for PsmCas13b and h25 for Ba1Cas12a3. For one-pot reactions, nucleases, crRNAs and fluorescent reporters were first combined in a single mixture. Target RNAs were then added to initiate the reactions. Fluorescence was monitored over time. Initial reaction velocities were calculated in the linear range. All reactions were performed in at least four independent replicates.
Microscale thermophoresis measurements
Microscale thermophoresis measurements were performed as previously described73 in buffer containing 40 mM Tris-HCl (pH 7.5), 50 mM NaCl, 2 mM MgCl2, 1 mM DTT and 1.66% glycerol. To pre-assemble the activated ternary complex, the purified components were mixed at equimolar ratios before preparing the dilution series. For all measurements, a 16-point 2-fold serial dilution series was prepared, starting at an initial concentration of 2.5 μM of unlabelled components. Each dilution was subsequently mixed in equal volumes with a constant concentration of Cy5-labelled RNA. Samples were transferred into premium-coated capillaries (MO-K025, NanoTemper Technologies), and microscale thermophoresis measurements were conducted using a Monolith Pico instrument (NanoTemper Technologies). Data analysis, including determination of Kd values, was performed using NanoTemper Analysis software and plotted using Origin software (OriginLabs).
Sample preparation for cryo-EM
Carboxy-terminal His-tagged Ba1Cas12a3 (CBS-7171) was overexpressed in E. coli (DE3) star in 4 l of LB medium. The cell culture was induced with 0.1 mM IPTG at an OD600 of 0.6 and further grown at 18 °C for 18 h. Cell pellets were collected by centrifugation and resuspended in 100 ml lysis buffer (50 mM Tris pH 7.5, 300 mM NaCl, 2 mM MgCl2, 10 mM imidazole and 10% glycerol) treated with 1 tablet of protease inhibitor cocktail (11873580001, Roche). Cells were disrupted by sonication (Amplitude 58%; 1 s on and 8 s off for 30 min total). The soluble fraction was obtained by centrifugation and incubated with 5 ml (bed volume) nickel resin rotating for 1 h at 4 °C. Subsequently, the purification procedure was conducted in a 20-ml gravity column. Nickel resin was washed with 50 ml wash buffer A (50 mM Tris pH 7.5, 2 M NaCl, 2 mM MgCl2, 10 mM imidazole and 10% glycerol) and 50 ml of wash buffer B (50 mM Tris pH 7.5, 50 mM NaCl, 2 mM MgCl2, 10 mM imidazole and 10% glycerol) sequentially and eluted with nickel-elution buffer (50 mM Tris pH 7.5, 50 mM NaCl, 2 mM MgCl2, 300 mM imidazole and 10% glycerol). Nickel peak elution fractions were collected and diluted 10 times with low-salt buffer (50 mM Tris pH 7.5, 20 mM NaCl, 2 mM MgCl2 and 10% glycerol). Protein samples were then loaded onto a HiTrap SP HP cation-exchange chromatography column using low-salt buffer and eluted with a gradient of high-salt buffer (50 mM Tris pH 7.5, 1.0 M NaCl, 2 mM MgCl2 and 10% glycerol). Peak elution fractions were pooled and loaded onto Superdex 200 increase 10/300 GL (GE Healthcare) using SEC buffer (50 mM Tris pH 7.5, 150 mM NaCl, 2 mM MgCl2 and 5% glycerol). Peak fractions were then pooled and concentrated, and flash-frozen in aliquots in liquid nitrogen.
The tRNA for cryo-EM was produced using the T7 RNA polymerase-mediated run-off method74. In detail, the in vitro transcription reaction was performed in a 500 µl volume containing DNA template, T7 RNA polymerase and reaction buffer (20 mM Tris, pH 8.0, 5 mM DTT, 150 mM NaCl, 8 mM MgCl2, 2 mM spermidine, 20 mM NTPs, RNasin and pyrophosphatase). The reaction was performed at 37 °C overnight and followed by DNase I treatment to remove DNA templates. The product was then purified using a DEAE column and heat treatment at 80 °C for 2 min and followed by the slow cooling process to room temperature for the re-annealing process. To obtain a homogenous tRNA population, the samples were subjected to a Superdex 75 Increase gel filtration column, and the tRNA-containing fractions were pooled and stored at −80 °C. For microscale thermophoresis measurements, the internally Cy5-labelled in vitro-transcribed E. coli tRNAs were produced as described above, with an additional 5% of Cy5-CTP introduced in the reaction.
To obtain the Ba1Cas12a3 quaternary complex, crRNA was pre-treated by heating at 65 °C for 3 min and slowly cooled down to room temperature. RNA was then added to Ba1Cas12a3 in a 1:1.2 molar ratio. The complex was then incubated at room temperature for 10 min. The pure Ba1Cas12a3 binary complex was purified via Superdex 200 Increase 10/300 GL in buffer without MgCl2 to inhibit nuclease activity (50 mM Tris pH 7.5, 50 mM NaCl, 1 mM DTT and 5% glycerol). Target RNA and tRNAAla were sequentially added to 50 μl of the Mg2+-free peak fraction of the binary complex (about 20 μM) at 1:1.2 and 1:2 molar ratios, respectively. The target RNA was incubated at room temperature for 10 min, followed by the addition of tRNA, and the mixture was kept on ice for 30 min before vitrification.
For the binary complex dataset, the Ba1Cas12a3 binary complex was prepared as above but with 2 mM MgCl2 present (50 mM Tris pH 7.5, 150 mM NaCl, 1 mM DTT, 2 mM MgCl2 and 5% glycerol). The binary complex sample was vitrified in the presence and absence of 0.02% (w/v) fluorinated octyl maltoside to obtain more particles with different orientations.
For the ternary complex dataset, target RNA was added into the Ba1Cas12a3 binary complex (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM DTT, 2 mM MgCl2 and 5% glycerol) in a 1:1.2 molar ratio. The ternary complex sample was vitrified in the presence of 1 mM ATPγS to obtain more views of particle distribution.
Cryo-EM data acquisition and processing
Samples were vitrified on cryo-EM grids (Quantifoil R 2/1 300 mesh, Au). In brief, 3.5 μl of the sample was applied onto glow-discharged grids. The grids were blotted for 4 s (blot force: −9; 100% humidity; 4 °C) and plunge-frozen in liquid ethane using a Vitrobot Mark IV (FEI). The vitrified specimens were imaged on a Thermo Fisher Glacios Cryo-TEM operating at 200 kV and equipped with the Falcon 4i Direct Electron Detector camera, an energy filter slit width of 10 eV and a C2 aperture of 50 μm. The videos were recorded in counting mode using Thermo Fisher Scientific EPU software (v.3.7.1) with the total dose of 40 e– A–2 with a nominal magnification of 130,000-fold, corresponding to a pixel size of 0.91 Å.
Motion correction, contrast transfer function estimation, particle picking and 2D classification were carried out on-the-fly using CryoSPARC live (v.4.4.0 and v.4.6.0)28,75. The particles selected from the 2D classification of the live session were divided into small splits and the junk particles were removed again using 2D classification. The remaining particles were further classified by using heterogeneous refinement, and the best 3D classes were subjected to non-uniform refinement. Overall, gold-standard resolution Fourier shell correlation using the threshold of 0.143 and local resolution was calculated by local resolution estimation. The statistics for data collection and processing are provided in Extended Data Table 1.
Model building, refinement and figure preparation
All models corresponding to the Ba1Cas12a3 binary, ternary and quaternary complexes were originally generated by AlphaFold3 (ref. 76) and used as initial models for model building. Models were built in ChimeraX (v.1.7)77, and interactive refinements against cryo-EM maps were performed using ISOLDE with restraints for secondary-structure elements of the AlphaFold3-predicted structures77,78. The resulting models were further refined with real-space refinement and validated in Phenix (v.1.20.1)79. Statistics for the final models are described in Extended Data Table 1.
All structural figures were generated using Chimerax (v.1.7). For structural comparison, the structures from binary to quaternary were aligned against the Ba1Cas12a3 WED domain. The domain motions from other individual domains were shown with arrows in different domain colours, and the arrows from each two compared models were created by using the PDB-arrows script with slight modification80.
Generation and purification of Ba1Cas12a3 mutants
The Ba1Cas12a3 mutant constructs were generated either by Q5 PCR site-directed mutagenesis followed by KLD or by Gibson assembly, using the C-terminal 6×His-tagged WT Ba1Cas12a3 plasmid as a template (CBS-7171; Supplementary Table 2). Each plasmid was transformed into E. coli BL21 (DE3) cells, grown on an agar plate and selected by antibiotic resistance selection. A single colony was used to inoculate 60 ml of LB medium, grown at 37 °C at 200 rpm overnight (16–18 h). Next, 20 ml of the overnight growth was used to inoculate 1 litre of LB medium containing 100 µg ml–1 kanamycin. Cells were grown at 37 °C at 200 rpm until an OD600 of 0.5–0.6 was reached. The cells were then cold shocked on ice for 20 min before inducing with 0.1 mM IPTG, followed by 16–18 h of growth at 18 °C. Cell pellets were collected by centrifugation and stored at −80 °C. Other nucleases were expressed using the same procedure.
For in vitro cleavage experiments shown in Fig. 3f,h,i, Extended Data Fig. 2 and Supplementary Figs. 11 and 13, Ba1Cas12a3, mutants thereof, as well as ApCas12a4, MpCas12a2 and Sm3Cas12a3, were purified similarly to the method used for SuCas12a2 (refs. 27,28). In brief, the cell pellets were thawed on ice before lysis by sonication in a lysis buffer (25 mM Tris pH 8.5, 500 mM NaCl, 10 mM imidazole, 2 mM MgCl2and 10% glycerol) containing protease inhibitors (2 μg ml−1 aprotinin, 10 μM leupeptin and 1.0 μg ml−1 pepstatin) and 1 mg ml−1 lysozyme. The lysate was clarified by centrifugation and added to 5 ml of Ni-NTA resin and batch bound at 4 °C for 30 min, and then washed with 300 ml wash buffer (25 mM Tris pH 8.5, 2 M NaCl, 10 mM imidazole, 2 mM MgCl2 and 10% glycerol). The protein was eluted with 25 ml Ni-elution buffer (25 mM Tris pH 8.5, 500 mM NaCl, 250 mM imidazole, 2 mM MgCl2 and 10% glycerol). Fractions containing Ba1Cas12a3, as determined by SDS–PAGE, were desalted using a Hiprep 26/10 desalting column into low-salt buffer (25 mM Tris pH 8.5, 50 mM NaCl, 2 mM MgCl2 and 10% glycerol). Ba1Cas12a3 was applied to a Hitrap Heparin HP cation-exchange column and eluted using a gradient of high-salt buffer (25 mM Tris pH 8.5, 1 M NaCl, 2 mM MgCl2 and 10% glycerol). The fractions containing Ba1Cas12a3 were concentrated using a 50 kDa MWKO concentrator to about 1 ml and loaded onto a Hiload 16/600 Superdex 200 pg size-exclusion column using SEC buffer (25 mM HEPES pH 8.5, 150 mM KCl, 2 mM MgCl2 and 5% glycerol). Fractions containing the desired proteins were concentrated and stored at −80 °C.
In vitro cleavage of truncated tRNAs
Reactions were made by combining 300 nM crRNA with 250 nM CRISPR-associated enzyme in DTT containing low-salt buffer (40 mM Tris-HCl pH 7.5, 50 mM NaCl, 2 mM MgCl2 and 1 mM DTT) and incubated at room temperature for 15 min. Next, 100 nM of FAM-labelled tRNA substrate was then added followed by 250 nM of target RNA to initiate the reaction. The reaction was performed at 37 °C for 1 h. Reactions were quenched with phenol and nucleic acid was purified by acid phenol–chloroform extraction. FAM-labelled nucleic acid was analysed using 12% urea–PAGE and visualized for fluorescein fluorescence.
For determining the cleavage efficiency of the Ba1Cas12a3 TRL mutants, 140 µl reactions were performed with 150 nM Ba1Cas12a3, 100 nM guide RNA, 100 nM FAM 26-nucleotide structured tRNA substrate (Supplementary Table 2) and 25 nM target RNA. Next, 20-µl aliquots were extracted and quenched at 10 s, 30 s, 1 min, 2 min, 5 min and 10 min at 37 °C. Nucleic acid was quenched and visualized as described above.
Orthologue target and substrate preference assay
For determining the nucleic acid targets for ApCas12a4, MpCas12a2, Sm3Cas12a3 and Ba1Cas12a3 (Extended Data Fig. 2), 250 nM crRNA and a given nuclease (250 nM) were incubated in a DTT containing low-salt buffer (40 mM Tris-HCl pH 7.5, 50 mM NaCl, 2 mM MgCl2 and 1 mM DTT) at room temperature for 15 min. Then 100 nM of FAM-labelled target (RNA, ssDNA or dsDNA) was added to initiate the reaction. The reaction was performed at 37 °C for 1 h before quenching the reaction with phenol and extracting the nucleic acid using phenol–chloroform. The samples were analysed using a previously described denaturing FDF–PAGE81 and visualized for fluorescein fluorescence.
Ba1Cas12a3 target electromobility shift assay
To determine whether the Ba1Cas12a3 ΔtRLD mutant could still bind target RNA, an electromobility shift assay was performed. To control the amount of binary (protein–guide) complex in the reaction mixture, 5:1 Ba1Cas12a3 to guide RNA concentration ratio was prepared. An initial protein–guide solution was prepared and serial diluted into four reaction concentrations in an EDTA-containing buffer (25 mM HEPES (pH 7.2), 150 mM KCL and 100 mM EDTA). The reaction tubes ranged from 10 nM to 10 µM for Ba1Cas12a3 and 2 nM to 2 µM for the crRNA. The FAM-labelled target RNA concentration was held constant at 100 nM. The samples were run on a 6% TBE polyacrylamide gel and visualized for fluorescein fluorescence.
Protein-folding analysis using circular dichroism spectroscopy
To determine the overall structural components of Ba1Cas12a3 ΔtRLD compared with WT Ba1Cas12a3, far-UV circular dichroism (CD) was performed using a Jasco-J1500 spectropolarimeter. In brief, 0.36 mg ml–1 of the respective protein was prepared in CD buffer (10 mM K2HOP4 and 50 mM Na2SO4 pH 8.74) and CD spectra were obtained from 260 to 190 nm using a scanning speed of 50 nm min−1 (with a 2-s response time and accumulation of three scans). The CD signal was converted to molar ellipticity using Jasco Spectra Manager software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.