Media and reagents
E. faecium was routinely cultured at 37â°C in brainâheart infusion (BHI) broth (Becton Dickson) or BHI agar (BHIA), BHI solidified with 1.5% agar (Becton Dickson). For electroporation, E. faecium was cultured in BHI supplemented with 3% glycine and 200âmM sucrose (pHâ7.0). Escherichia coli was cultured in Luria broth. Broth microdilution (BMD) MICs were performed in cation-adjusted Mueller Hinton with TES broth (CAMHBT) (Thermo Fisher Scientific). A concentration of 10âmgâlâ1 chloramphenicol (Sigma-Aldrich) was used for plasmid selection in E. faecium and E. coli. The following antibiotics were used at variable concentrations for susceptibility testing: rifampicin (Sigma-Aldrich), rifaximin (Sigma-Aldrich) and DAP (Cubicin).
Oligonucleotides were purchased from Integrated DNA Technologies and are listed in Supplementary Table 11. Plasmids were purified using the Monarch Plasmid Miniprep Kit (NEB). PCR products and gel extractions were purified using the Monarch DNA Gel Extraction Kit (NEB). Genomic DNA was purified using the Monarch Genomic DNA Purification Kit (NEB). Phusion and Phire DNA polymerase was purchased from NEB.
Bacterial isolates
A list of the bacterial strains used in this study is provided in Supplementary Table 10. Australian bacterial strains were collected across three data projects in the Microbiological Diagnostic Unit Public Health Laboratory (MDU PHL). Two unbiased cross-sectional surveys of VREfm were conducted between 10 November 2015 and 9 December 2015 (nâ=â331)26 and between 1 November 2018 and 30 November 2018 (nâ=â323) in the state of Victoria (referred to as the 2015 and 2018 snapshot, respectively). During this period, all VREfm-positive isolates (including screening and clinical samples) collected by laboratories across the state were sent to the MDU PHL. Moreover, this project included vanA-VREfm collected from the Controlling Superbugs study14,15, a 15-month (April 2017 to June 2017 and October 2017 to October 2018) prospective study including 8 hospital sites across 4 hospital networks, resulting in 346 VREfm isolates (308 patients) sent for WGS at MDU PHL. The VREfm were isolated from patient samples (including screening and clinical samples) routinely collected from hospital inpatients. For the âhistorical vanA-VREfm,â every vanA isolate collected within MDU PHL was included. This resulted in an additional 229 isolates, sampled between 2003 and 2014. Collection of bacterial isolates for this study was approved by the Melbourne Health Human Research Ethics Committee (HREC), endorsed by the corresponding HREC at each participating site (HREC/13/MH/326) and the University of Melbourne Human Research Ethics Committee (22536). Approvals included a waiver of consent in accordance with the National Statement on Ethical Conduct in Human Research 2007 (Australia).
For publicly available isolates, our aim was to capture the diversity of E. faecium circulating globally by including isolates that formed part of several key studies involving hospital-associated VREfm (as of January 2021). To be included, isolates needed to have short-read data available, with geographical location (by country), year of collection and source (human or animal). Reads were included only if they had a sequencing depth of >50Ã. To capture the diversity of VREfm circulating in the USA, isolates from human sources were downloaded from the PathoSystems Resource Integration Center32. All isolates were confirmed to be E. faecium with the Kraken2 database (v.2.1.2)33. The final number of international isolates comprised those from Africa (nâ=â8), Asia (nâ=â25), Europe (nâ=â2,941), North America (nâ=â424) and South America (nâ=â78) (Supplementary Table 10).
Antibiotic susceptibility testing
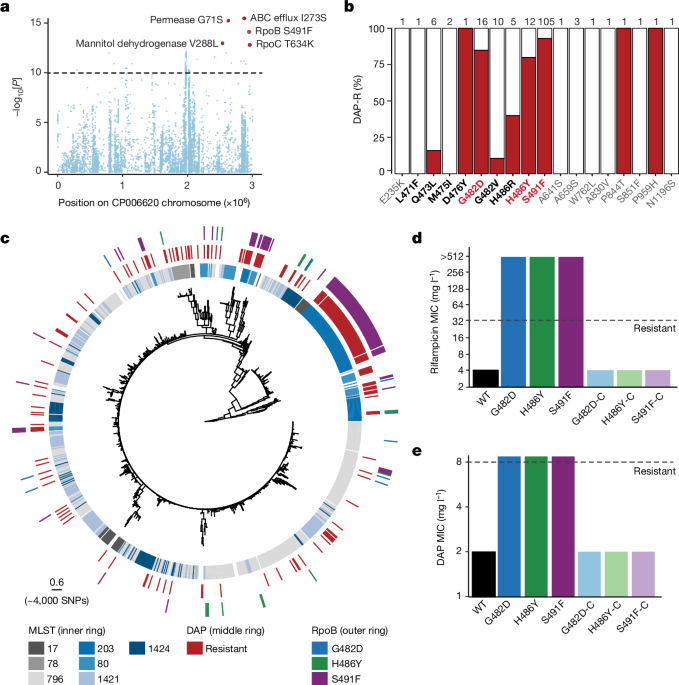
Daptomycin susceptibility testing was performed using the BMD MIC method according to CLSI guidelines. In a 96-well plate, a twofold dilution series (from 32 to 0.5âmgâlâ1) of DAP was made in 100âμl volumes of CAMHBT, additionally supplemented with 50âmgâlâ1 Ca2+. An inoculum of 100âμl E. faecium broth culture adjusted to 1âÃâ106 colony-forming units (CFU) per ml in CAMHBT was then added to each well. After incubation for 24âh, the MIC was defined as the lowest antimicrobial concentration that inhibited visible growth. All assays were performed in biological triplicate, with the median MIC reported. In accordance with recent guidelines34, isolates with a DAP MICââ¥â8âmgâlâ1 were considered to be DAP-R. A DAP-sensitive strain (AUS0085)35 and a DAP-R strain (DMG1700661)36 were used as controls.
Rifampicin susceptibility testing was performed using the BMD method in CAMHBT. High-level rifampicin resistance was defined with a MICâ>â32âmgâlâ1. All susceptibility testing was performed in triplicate.
WGS analysis
Genomic DNA was extracted from a single colony using the JANUS automated workstation (PerkinElmer) and Chemagic magnetic bead technology (PerkinElmer). Genomic DNA libraries were prepared using the Nextera XT kit according to the manufacturerâs instructions (Illumina). WGS was performed using the Illumina NextSeq platform, generating 150âbp paired-end reads. The short reads of isolates sequenced at MDU-PHL are available at the NCBI Sequence Read Archive (BioProject: PRJNA565795 (controlling superbugs), PRJNA433676 (2015 snapshot) and PRJNA856406 (2018 snapshot) and PRJNA856406 (historical vanA isolates)).
Phylogenetic analysis
De novo assemblies of the genomes were constructed using Spades37 (v.3.13). In silico MLST was determined using the program mlst with the efaecium database38 (https://github.com/tseemann/mlst). The 1,000 Australian genomes as well as the 4,705 Australian and international VREfm were mapped to the reference E. faecium genome AUS0085 isolated from a human bacteraemia infection in Victoria, Australia (NCBI: CP006620)35 using snippy (https://github.com/tseemann/snippy) (v.4.4.5), applying a minfrac value of 10 and mincov value of 0.9. This reference was selected as it was a publicly available complete genome collected locally and DAP-S. A maximum-likelihood phylogenetic tree was inferred using IQ-TREE (v.2.1.2)39 with a general time-reversible (GTRâ+âG4) substitution model, including invariable sites as a constant pattern and 1,000 bootstrap replicates. Recombination masking was not performed for species maximum-likelihood trees due to the small size of the resulting core alignment. All trees were mid-point rooted and visualized in R (v.4.0.3; https://www.r-project.org/) using phangorn40 (v.2.5.5), ape41 (v.5.4), ggtree42 (v.2.3.4) and ggplot (v.3.3.2).
The genome assemblies of all isolates were screened for acquired AMR determinants using abriTAMR (https://github.com/MDU-PHL/abritamr)43.
GWAS analysis of DAPÂ resistance
A GWAS approach was applied to identify genetic variants of DAP resistance in E. faecium. A genotype matrix of SNPs was constructed and used as input to homoplasyFinder44 (v.0.0.0.9) to determine the consistency index at each locus and kept mutations that had an index of â¤0.5 (indicating at least two independent acquisitions across the phylogeny). We then ran GWAS using DAP resistance as a binary trait, where isolates were categorized as resistant if their DAP MIC was â¥8âmgâlâ1. To correct for population structure, we used the factored spectrally transformed linear mixed models (FaST-LMM) implemented in pyseer45 (v.1.3.6), which computes a kinship matrix based on the core genome SNPs as a random effect. P values were corrected for multiple-hypothesis testing using the Bonferroni correction method.
Competition assay
For competition assays, overnight cultures of WT and corresponding RpoB(S491F) or RpoB(S491F)/RpoC(T634K) mutants were diluted to an optical density at 600ânm (OD600) of 0.5 in BHI and equal volumes added to an overnight culture. Serial dilutions of each co-culture were performed at times 0 and 24âh on BHIA. Colonies were then replica plated onto BHIA and BHIA rifampicin 20âµgâmlâ1 to determine the proportion of WT to mutant.
cgMLST and clustering
cgMLST alleles for each isolate were defined using the public E. faecium cgMLST scheme46 and chewBBACA (v2.0.16)47, implemented locally in the COREugate pipeline (v.2.0.4) (https://github.com/kristyhoran/Coreugate). The pipeline determines the alleles of each core gene for every isolate as defined by the specific pathogen scheme. The E. faecium cgMLST scheme contains 1,423 genes. The number of allelic differences between each isolate within this core set of genes is then determined. The cgMLST clusters were determined using single linkage clustering and a pairwise allelic difference threshold of â¤250. This threshold was chosen as it maximized diversity within clusters, to improve temporal sampling depth, while still clustering based on maximum-likelihood tree structure.
Phylodynamic analysis of the emergence of the S491F(RpoB) mutation in VREfm lineages
To investigate the emergence of the S491F mutation in RpoB in three different lineages, as defined with cgMLST, we undertook further analysis on these clusters/lineages. From the species-level maximum-likelihood tree (Fig. 2c), three lineages/clusters were identifiable by cgMLST due to their size (nâ>â50) and presence of the S491F mutation. The three clusters were analysed independently, such that individual core-genome SNP alignments were generated, as this increased the length of the core alignment and number of sites considered. Snippy (https://github.com/tseemann/snippy) (v.4.4.5) was used to generate the alignments for each cluster to the corresponding reference genome (AUSMDU00004024 (CP027517.1) for cluster 1; AUSMDU00004055 (CP027506.1) for cluster 2; and AUSMDU00004142 (CP027501.1) for cluster 3). Each core alignment used a within âcluster referenceâ (complete genome of the same cluster) to maximize core-SNP alignment length. The reference for each cluster was chosen as they were a locally collected closed genome. Recombination was removed from the final alignment using Gubbins48 (v.2.4.1) to ensure that modelling was informed only by SNPs with tree-like evolution within the core genome. Maximum-likelihood trees for each of the three clusters were inferred from the core-SNP alignments (cluster 1, nâ=â219 taxa, 329 SNPs; cluster 2, nâ=â85 taxa, 541 SNPs; cluster 3, nâ=â68 taxa, 764 SNPs) with IQ-tree (v.2.1.2)49 with a general time-reversible (GTRâ+âG4) substitution model, including invariable sites as a constant pattern. Phylogenetic uncertainty was determined through 1,000 nonparametric bootstrap replicates.
To investigate temporal signal in the three clusters of VREfm genomes, we first used TempEst50 (v.1.5). A root-to-tip regression analysis was performed on the root-to-tip branch distances within the three, cluster maximum-likelihood phylogenies as a function of year of collection, with the position of the root optimized according to the heuristic residual mean squared method.
The frequency of the emergence of the rpoB mutation in VREfm was inferred using a discrete trait model implemented in BEAST51 (v.1.10.4). Under this model the SNP alignments are used to infer the evolutionary process (that is, phylogenetic tree, time and nucleotide substitution model parameters) for the three clusters. The alignments all shared the HKY substitution model and a constant-size coalescent population prior19. To avoid ascertainment bias due to using a SNP alignment, the number of constant sites was considered for the likelihood calculations. The molecular clock was a relaxed clock with an underlying lognormal distribution. The molecular clock was calibrated using isolation dates for each genome by year of collection and the mean clock rate is shared between all three alignments, but the model allows for the individual alignments to have different standard deviations of the log-normal distribution and also different branch rates. The mean molecular clock rate requires an explicit prior distribution, for which we used a Gamma distribution and a 0.95 quantile range of 4.9âÃâ10â6 and 1.1âÃâ10â4 substitutions per site per year. This informative prior means that it acts as an additional source of molecular clock calibration. The median substitution rate was similar for cluster 1 and cluster 2, at 9.7âÃâ10â7 (95% highest posterior density (HPD) 6.88âÃâ10â7â1.24âÃâ10â6) and 1.25âÃâ10â6 (95% HPD 7.68âÃâ10â7â1.74âÃâ10â6), respectively, but slightly faster for cluster 3 at 3.86âÃâ10â6 (95% HPD 2.23âÃâ10â6â5.69âÃâ10â6). Cluster 1 (nâ=â219) was inferred from a core alignment of 1,869,554âbp containing 329 SNP sites; cluster 2 (nâ=â85) was inferred from an alignment of 1,524,024âbp containing 541 SNP sites; and cluster 3 (nâ=â68) was inferred from an alignment of 1,860,780âbp containing 764 SNP sites.
The presence or absence of the S491F mutation in rpoB was used as a binary trait52,53. The trait model was shared between the three alignments, with the different Markov jumps and rewards (that is, changes of trait state and time spent in each state, respectively) recorded for each of the three alignments. The posterior distribution of model parameters was sampled using a Markov chain Monte Carlo of 100,000,000 iterations, sampling every 100,000 iterations. Two independent runs were run for the models. We assessed sufficient sampling from the stationary distributions by verifying the effective sample size of key parameters was around or above 200. The final MCC trees were visualized in R (v.4.0.3, https://www.r-project.org/) using ggtree42 (v.2.3.4). The Markov jumps for the rpoB trait for each alignment were visualized in R (v4.0.3, https://www.r-project.org/).
Construction of isogenic mutants using allelic exchange and of pRAB11prdR
The rpoCT634K, rpoBG482D, rpoBH486Y, rpoBS491F, rpoBQ473L, ABC transporter (I274S), permease protein (G71S) or mannitol dehydrogenase (V288L) mutations were recombined into the chromosomal copy of each gene in ST796 VREfm (Ef_aus0233) by allelic exchange. Deletions of the CpsA, K+ transporter, hypothetical protein, DltC or PrdRAB were also completed using allelic exchange. The region encompassing each gene was amplified by splice overlap extension (SOE)-PCR and recombined into pIMAY-Z54 using the seamless ligation cloning extract (SLiCE)55 method and transformed into E. coli IM08B54. The construct was transformed into electrocompetent VREfm55, with allelic exchange performed as described previously56. Reversion of rpoBG482D, rpoBH486Y or rpoBS491F mutations were completed using allelic exchange with a construct containing the respective wild-type allele. To construct a vector containing prdR, the vector pRAB11 was used. The prdR gene was amplified using Aus0233 genomic DNA. The prdR product was gel extracted, SLiCE cloned into amplified pRAB11, and transformed into IM08B, yielding pRAB11:prdR. The plasmid and EV were then electroporated into Aus0233.
Genome sequencing and analysis of all mutants was conducted as described, with resulting reads mapped to the Ef_aus0233 reference genome and mutations identified using Snippy (https://github.com/tseemann/snippy) (v.4.4.5).
VREfm in vivo gastrointestinal colonization experiments
Female C57BL/6 mice at 6â8 weeks of age were purchased from WEHI and maintained in a specific-pathogen-free facility at the Peter Doherty Institute for Infection and Immunity. The facility operates a 12âhâ12âh lightâdark cycle and maintains ambient temperature (18â23â°C) and humidity (40â60%). Animals were administered a standard mouse chow diet (Barastoc irradiated mouse cubes) and provided with water ad libitum. All animal handling and procedures were performed in a biosafety class 2 cabinet. Animal procedures were performed in compliance with the University of Melbourne guidelines and approved by The University of Melbourne Animal Ethics Committee (application IDs: 20094 and 28528). Animals were randomly assigned into cages on reception. After acclimatization, the cages were randomly assigned to treatment groups.
Experimental group sizes (treatment versus controls) were calculated using a power of 80%, an attrition rate of 15% and a type I error of 5%. The dose for each antibiotic was calculated using the US Food and Drug Administration (FDA) human conversion formula to ensure that each mouse was given a human-equivalent dose57. To establish gastrointestinal colonization of VREfm, mice were administered ceftriaxone (410âmgâkgâ1 dayâ1; AFT Pharmaceuticals) through subcutaneous injection once daily for 4 days, followed by an antibiotic wean period of 24âh. Mice were then inoculated with 106 VREfm in 100âμl PBS by oral gavage. Then, 3âdays after VREfm inoculation, single-housed mice were administered either rifaximin (113âmgâkgâ1 administered twice daily; Sigma-Aldrich), rifampicin (123âmgâkgâ1 administered once day; Sigma-Aldrich) or vehicle (Corn oil with 10% DMSO) through oral gavage; or DAP (50âmgâkgâ1 administered once daily; Cubicin) through subcutaneous injection (this results in similar exposure (AUC0â24) to that observed in humans receiving 8âmgâkgâ1 of intravenous DAP58). The above antibiotic dosing protocol was followed for 7âdays. Faecal samples were collected at specific timepoints throughout the experiment to determine VREfm gut colonization and for downstream rifamycin and DAP resistance analysis. Investigators were blinded to treatment groups with faecal samples de-identified on collection from individual mice before being resuspended in PBS to a normalized concentration (100âmgâmlâ1). Serial dilutions of each de-identified faecal sample were performed, and the samples were plated onto Brilliance VRE agar (Thermo Fisher Scientific) for VREfm CFU enumeration.
For rifamycin and DAP analysis, VREfm colonies (nâ=â50 per de-identified faecal sample per mouse) from the Brilliance VRE agar plates were replica plated onto BHIA with and without rifampicin 20âµgâmlâ1 to determine the proportion of rifampicin-resistant VREfm in each mouse. Fifty colonies per mouse were then screened for DAP resistance, with a single colony being resuspended in PBS, then diluted 1/100 into CAMHBT containing 50âmgâlâ1 Ca2+ and 1/100 in CAMHBT containing 50âmgâlâ1 Ca2+ and 8âmgâlâ1 DAP. All suspected DAP-R colonies were confirmed using a DAP BMD MIC as before.
To determine which mutations were present in the rifamycin-resistant isolates, a random selection of 300 colonies, 150 from rifaximin-treated mice and 150 from rifampicin-treated mice (50 pre and 100 post for each treatment), were sampled for WGS as described above.
Analysis of patients receiving rifaximin for hepatic encephalopathy prophylaxis
To examine the potential association between rifaximin exposure and DAP-R VREfm, we analysed VREfm collected between 2014 and 2022 from a single quaternary hospital institution in Melbourne. In total, 225 patients were assessed for previous exposure to rifaximin, which was defined as at least a single dose administered before the collection date for the VREfm isolate and grouped into a rifaximin-exposed group and an unexposed control group. Only a single isolate was selected at random for testing and analysis from patients who had multiple samples with VREfm isolates. The VREfm isolates underwent WGS and DAP and rifampicin susceptibility testing as before. Patients with VREfm isolates that were assessed as genetically clustered with other VREfm isolates in the cohort and likely represented direct transmission were excluded. Genetic clustering was defined using an international standard SNP cut-off (7 SNPs)59,60 using a split k-mer (kâ=â15) analysis (https://github.com/simonrharris/SKA) (v.1.0), a reference-free pairwise method that compares the entire genome (unlike traditional core-genome based comparisons). Medical records from patients were reviewed for comorbidity and antibiotic prescribing data. Potential associations were assessed through univariate analysis using Pearsonâs Ï2 tests or Fisherâs exact tests for categorical data, and Studentâs t-tests (parametric) or Wilcoxon rank-sum tests (nonparametric) for continuous data. To determine predictors of DAP-R VREfm, multivariable logistic regression with backward stepwise elimination procedure was used, excluding variables with Pâ>â0.10 and reincluding variables with Pâ<â0.05. Exposure to rifampicin and DAP were forced into the models as variables a priori. A P value of <0.05 was considered to be statistically significant. Cases with missing data (for example, incomplete medical record data due to interhospital transfer) were excluded. Several sensitivity analyses were also performed to assess for independent associations after excluding potential confounders, including analysis with exclusion of variables with <10 outcomes, assessing associations with antimicrobial exposure separately from demographic and comorbidity data, and modifying the rifaximin exposure variable to include (1) any previous exposure to rifaximin (including both recent and distant exposure), and (2) any previous exposure to rifamycin antibiotics (including rifampicin, rifabutin and rifaximin). The genomic relationships of VREfm isolates were visualized in a maximum-likelihood phylogenetic tree as before, using a core-SNP alignment of 12,886 sites. The mutations in RpoB were determined using snippy (https://github.com/tseemann/snippy) (v.4.4.5) as described.
Data were obtained from medical records with approval from the Austin Health Human Research Ethics Committee (HREC/92971/Austin-2023), which included a waiver of consent in accordance with the National Statement on Ethical Conduct in Human Research 2023 (Australia).
Analysis of patients receiving rifaximin for HSCT prophylaxis
To examine the potential association between rifaximin exposure and the presence of DAP-associated rpoB substitutions in VREfm independent of underlying chronic liver disease, we analysed isolates collected from patients undergoing HSCT from a hospital institution in Regensburg, Germany. In this cohort, rifaximin is used for gut decontamination to reduce the risk of gastrointestinal graft-versus-host disease. Liver cirrhosis is a contraindication to HSCT. Notably, no patient received prophylactic DAP treatment, which is a major risk factor for DAP-R VREfm. There were 68 patients initially assessed for recent exposure to rifaximin, which was defined as at least a single dose administered within 90 days before the isolate collection date. Only a single isolate was retained for testing and analysis from patients who had multiple samples. In this instance, the isolate included was randomized. The isolates underwent WGS using ion-torrent next-generation sequencing technology (Thermo Fisher Scientific) and Nanopore sequencing (Oxford Nanopore). Patients with isolates that were assessed as genetically clustered with other isolates in the cohort and likely represented direct transmission were excluded (as above). Statistical and phylogenetic analyses were undertaken as described above.
Data were obtained from medical records with approval from the local ethics committee (ethical committee of the University of Regensburg, 21-2521â101). Stool samples were collected from patients after obtaining written informed consent, and the study was performed in accordance with the Declaration of Helsinki.
Lipidomic analyses
Cultures of VREfm (nâ=â5) were grown to mid-exponential phase (OD600â=â0.6) and washed in PBS. The protein content for each sample was measured using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) and was normalized to 100âμg. Cells were lysed using the Bertin Precellys 24 homogenizer set at 6,000ârpm for 40âs and lipids were subjected to monophasic extraction as described previously61. Lipidomic samples were analysed using ultra-high-performance liquid chromatography (UHPLC) coupled to tandem mass spectrometry (MS/MS) using the Vanquish UHPLC system linked to an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific), with separate runs in positive- and negative-ion polarities. Solvent A comprised 60:40 (v:v) acetonitrile/water with 5âmM medronic acid and 10âmM ammonium acetate and solvent B comprised 90:10 (v:v) isopropanol:acetonitrile with 10âmM ammonium acetate. 10âµl of each sample was injected into the Acquity UPLC HSS T3 C18 column (1âÃâ150âmm, 1.8âµm; Waters) at 50â°C at a flow rate of 60âμlâminâ1 for 3âmin using 3% solvent B. During separation, the percentage of solvent B was increased from 3% to 70% in 5âmin and from 70% to 99% in 16âmin. Subsequently, the percentage of solvent B was maintained at 99% for 3âmin. Finally, the percentage of solvent B was decreased to 3% in 0.1âmin and maintained for 3.9âmin.
All MS experiments were performed using an electrospray ionization source. The spray voltages were 3.5âkV in positive-ionization mode and 3.0âkV in negative-ionization mode. In both polarities, the flow rates of sheath, auxiliary and sweep gases were 25 and 5 and 0 arbitrary unit(s), respectively. The ion-transfer tube and vaporizer temperatures were maintained at 300â°C and 150â°C, respectively, and the ion funnel RF level was set at 50%. In the positive-ionization mode from 3 to 24âmin, a top-speed data-dependent scan with a cycle time of 1âs was used. Within each cycle, full-scan MS spectra were acquired first in the Orbitrap at a mass resolving power of 120,000 (at m/z 200) across an m/z range of 300â2,000 using quadrupole isolation, an automatic gain control (AGC) target of 4âÃâ105 and a maximum injection time of 50âms, followed by higher-energy collisional dissociation-MS/MS at a mass resolving power of 15,000 (at m/z 200), a normalized collision energy (NCE) of 27% in positive mode and 30% in negative mode, an m/z isolation window of 1, a maximum injection time of 35âms and an AGC target of 5âÃâ104.
Identification and quantification of lipids and statistical analysis
LCâMS/MS data were searched using MS Dial v.4.90. The mass accuracy settings were 0.005âDa and 0.025âDa for MS1 and MS2. The minimum peak height was 50,000 and the mass slice width was 0.05âDa. The identification score cut-off was 80%. In positive-ionization mode, [Mâ+âH]+, [Mâ+âNH4]+ and [Mâ+âH-H2O]+ were selected as ion forms. In negative-ionization mode, [M-H]â and [Mâ+âCH3COO]â were selected as ion forms. All lipid classes available were selected for the search. PC, Lys-PC, DG, TG, CE and SM were identified and quantified from positive-ionization mode while PE, LPE, PS, LPS, PG, LPG, PI, LPI, PA, LPA, Cer and CL were identified and quantified in negative-ionization mode. The retention-time tolerance for alignment was 0.1âmin. Lipids with a maximum intensity of less than fivefold of average intensity in blank were removed. All other settings were set as the default. All lipid LCâMS features were manually inspected and reintegrated when needed. These four types of lipids, (1) with only sum composition except SM, (2) lipids identified due to peak tailing, (3) retention time outliner within each lipid class, (4) LPA and PA artefacts generated by in-source fragmentation of LPS and PS, were also removed. The shorthand notation used for lipid classification and structural representation follows the nomenclature proposed previously62. Relative quantification of the lipid species was achieved using the MS intensity of each lipid ion at apex of the LC peak and normalized to the protein quantity in each sample.
RNA-seq transcriptomics analysis
Cultures were grown to mid-exponential phase (OD600â=â0.6) and total RNA was extracted using the Direct-zol RNA Miniprep kit (Zymo Research) according to the manufacturerâs instructions. Cells were lysed using the Bertin Precellys 24 homogenizer set at 6,000ârpm for 40âs. The samples were treated with TURBO DNase (Thermo Fisher Scientific) followed by clean-up using the RNA clean and concentrator kit (Zymo Research) according to the manufacturerâs instructions. The absence of DNA contamination was checked by PCR and RNA integrity and purity was checked using the Bioanalyser RNA kit (Agilent). Five sequencing libraries from independent RNA extractions were made for each of the VREfm strains using the Stranded Total RNA with Ribo-Zero Plus (Illumina) kit and sequenced on a single lane of the Illumina NovaSeq 6000 platform. Raw paired-end reads were quality trimmed using TrimGalore (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) (v.0.6.2). Bases with a quality score <20 and reads shorter than 50âbp after trimming were discarded. rRNA was removed by the BBDuk script in BBtools (https://sourceforge.net/projects/bbmap/) (v.39.01). The resulting reads were aligned to the Aus0233 reference genome by Bowtie263 (v.2.5.1) using the –no-mixed flag and read counts were generated using htseq-count64 (v.0.12.4) using the options -r pos -t CDS -m union –nonunique none. Differentially expressed genes were detected using Degust (v.4.1.1). Genes with log2[fold change]â>â1.5 and adjusted Pâ<â0.05 were considered differentially expressed.
Proteomic analysis
Pelleted snap-frozen bacterial cells (OD600â=â0.6) were solubilized in 4% SDS, 100âmM Tris pHâ8.5 by heating them for 10âmin at 95â°C. The protein concentrations were assessed by a bicinchoninic acid protein assay (Thermo Fisher Scientific) and 100âµg of each biological replicate prepared for digestion using S-trap Mini Columns (Protifi) according to the manufacturerâs instructions. In brief, the samples were reduced with 10âmM DTT for 10âmin at 95â°C and then alkylated with 40âmM IAA in the dark for 1âh. The samples were acidified to 1.2% phosphoric acid and diluted with seven volumes of S-trap wash buffer (90% methanol, 100âmM tetraethylammonium bromide pHâ7.1) before being loaded onto S-traps and washed three times with S-trap wash buffer. The samples were then digested with trypsin before being collected by centrifugation after the addition of 100âmM tetraethylammonium bromide, followed by 0.2% formic acid and then 0.2% formic acid/50% acetonitrile. The samples were dried and further cleaned up using C18 Stage65,66 tips to ensure the removal of any particulate matter.
C18 enriched proteome samples were resuspended in 2% acetonitrile (aq) containing 0.01% trifluoroacetic acid (buffer A*) and separated using the Vanquish Neo UHPLC (Thermo Fisher Scientific) system with a single-column chromatography set up composed of a ACQUITY UPLC Peptide BEH C18 Column (300âà , 1.7âµm, 1âmmâÃâ100âmm, Waters) at a flow rate of 50âμlâminâ1. Proteome samples were loaded directly on to the ACQUITY column with buffer A (0.1% formic acid, 2% DMSO) coupled directly to an Orbitrap 480 mass spectrometer (Thermo Fisher Scientific) and the buffer composition altered from 2% buffer B (0.1% formic acid, 77.9% acetonitrile, 2% DMSO) to 26% B over 70âmin, then from 26% B to 99% B over 2âmin and then was held at 99% B for 1.5âmin. The mass spectrometer was operated in a data-independent mode automatically switching between the acquisition of a single Orbitrap MS scan (370â1,050âm/z, maximal injection time of 50âms, an AGC set to a maximum of 300% and mass resolving power of 120,000 (at m/zâ200) and the collection of 16.5âm/z DIA windows between 375 and 1,015âm/z (200â2,000âm/z, NCE 32%, maximal injection time of 54âms, an AGC set to 1,000% and a mass resolving power of 30,000 (at m/z 200). Identification and label free quantification (LFQ) analysis were accomplished using Spectronaut (Biognosys) v.16 (16.0.220606.53000) using directDIA based analysis with minor modifications: protein LFQ method set to MaxLFQ, single hit proteins excluded and imputation disabled. Data were searched against the E. faecium Aus0004 proteome35 (UniProt: UP000007591) with carbamidomethyl (C) allowed as a fixed modification and acetyl (protein N-term) as well as oxidation (M) allowed as variable modifications. Data outputs from Spectronaut were processed using Perseus (v.1.6.0.7)67 with missing values imputed based on the total observed protein intensities with a range of 0.3ââÏ and a downshift of 1.8âÏ. Statistical analysis was undertaken in Perseus using two-tailed unpaired t-tests and ANOVA. Proteins with log2[fold change]â>â1 and adjusted Pâ<â0.05 were considered to be differentially expressed.
Computational modelling
In predicting the potential effects of substitutions Q473L, G482D, H486Y and S491F, the full E. faecium DNA-dependent RNA polymerase was initially modelled using advanced homology modelling in Maestro (Schrodinger suite). BLAST-pdb was used to identify the M. tuberculosis homologue (PDB: 5UHC)68 as the template, as it had the best sequence identities across all RNA polymerase subunits. Modelling was performed based on the consensus between sequence alignments from MAFFT-DASH69, T-COFFEE70 and Clustal-W71 (within Maestro), which were manually optimized to minimize sequence gaps. The final RNA polymerase model, bound to rifampicin and the DNA replication fork was next subjected to loop refinement and minimization, and iteratively assessed for model quality within Maestro.
The modelled structure was used as input within in silico biophysical predictors Dynamut272, mmCSM-lig (https://biosig.lab.uq.edu.au/mmcsm_lig/), mmCSM-NA73 and mCSM-PPI273, which predicted the effects of mutations Q473L, G482D, H486Y and S491F on β-subunit stability, and affinities to rifampicin, nucleic acids within the replication fork, and other RNA polymerase subunits, respectively. During interpretation, all values were collectively considered to assess potential protein-level implications to wild-type function. In doing so, the affinity values for mutations located beyond 12âà of the binding partner were presumed negligible.
Estimation of zeta potential
The zeta potential was measured on cells grown to exponential phase (OD600â=â0.6) and washed in PBS. The zeta potential measurements were performed in PBS to minimize the influence of pH. Each experiment was performed under identical experiment conditions (nâ=â5), 25â°C with 2âmin of equilibration. The zeta potential was measured with a Zetasizer (Malvern).
Determination of cell-associated DAP with BoDipy labelling
BoDipy fluorescent dye (4,4-difluoro-1,3,5,7,8-pentamethyl-4-bora-3a,4a-diaza-S-indacene) (Invitrogen) was used to label DAP with minor modifications74. In brief, 50âμl DAP (50âmgâmlâ1) was mixed with 100âμl BoDipy (10âmgâmlâ1) and was made up to a final volume of 1âml in 200âmM sodium bicarbonate (pHâ8.5). The reaction was incubated for 1âh at 37â°C and unbound BoDipy was removed by dialysis at 4â°C using a Slide-A-Lyzer cassette (Thermo Fisher Scientific), with a 2.0âkDa cut-off according to the manufacturerâs instructions. The antibiotic activity of BoDipyâDAP was confirmed by BMD (described above). To measure cell-associated DAP, cultures were grown to exponential phase (OD600â=â0.6) 50âmgâlâ1 CaCl2. Each culture was incubated with BoDipyâDAP in darkness (10âmin) and washed to remove unbound BoDipyâDAP. The amount of bound BoDipyâDAP was measured with excitation at 490ânm and emission at 528ânm using an Ensight microplate reader (PerkinElmer). Biological replicates (nâ=â5) were completed on separate days.
Data visualization and statistics
All figures were generated in R (v.4.0.3, https://www.r-project.org/) using tidyverse (v.1.3.1), patchwork (v.1.1.1), ggnewscale (v.0.4.5) and maps (v.3.4.2). Statistical analyses were performed using R (v.4.0.3, https://www.r-project.org/) and GraphPad Prism (v.9.3.1). Specific tests are provided together with each corresponding result in the text.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.