Plant materials
The 402 tomato accessions used in this study are listed in Supplementary Table 8. Cultivated tomato lines, MM and M82, were used for the transgenic experiments. All of the tomato materials used in this study were grown in a greenhouse. The experimental plant materials in Figs. 2 and 4 and Extended Data Fig. 4b,e,h,i were grown in a greenhouse at the Beijing experimental station (40°â13â²â58.66â³âN, 116°â06â²â47.32â³âE, Beijing, China) in the autumn of 2018, and the spring of 2020, 2022 and 2023. The experimental plant materials in Extended Data Fig. 4a,d,f,g and Extended Data Fig. 4c were grown in a greenhouse at the Shouguang experimental station (36°â54â²â21â³âN, 118°â51â²â46â³âE, Shandong, China) in the autumn of 2021 and spring of 2020, respectively. Tomato seedlings were grown in a commercial nursery for 30â40 days and then transplanted to the greenhouse. The tomato plants were provided special care with adequate supply of water and fertilizer, and diseased plants were removed as soon as they were found. If the inflorescence was abnormally developed, a maximum of eight fruits were retained for each inflorescence. For the analyses of fruit yield, we collected all of the fruits from six subsequent individual inflorescences, until they were ripe. The fruit weight was determined per inflorescence. Total yield was the sum of fruit weight from each inflorescence for each plant. Plants that were diseased or grown in guard rows were marked and excluded from the analyses.
For fruit developmental analysis, the harvested fruit was divided into five categories, according to their maturity stage: immature green, mature green, breaker, orange and ripe based on the tomato colour chart USDA Visual Aid TM-L-1 (ref.â59). Only fruits that appeared developmentally equivalent were used for analysis. The pericarp of six fruits was excised, immediately frozen in liquid nitrogen, ground by means of a cryogenic mill and stored at â80â°C for further analysis. N. benthamiana used in this study was grown in a growth chamber with a light/dark photoperiod of 8/16âh at 25â°C.
Association mapping
We used the previously reported SNP dataset for the GWAS analysis7 with the EMMAX program60. The matrix of pairwise genetic distances was used as the varianceâcovariance matrix for random effects, and the first ten principal components were included as fixed effects. The genome-wide significance threshold was set as Pâ=â1/n, in which n is the effective number of independent SNPs. The effective number of independent SNPs was calculated using Genetic type 1 Error Calculator software61. The significant P value threshold was Pâ=â1.0âÃâ10â6. The Haploview software was used to calculate linkage disequilibrium, with the following parameters: -maxdistance 2,000 -minMAF 0.05 -hwcutoff 0 (ref.â62). Pairwise linkage disequilibrium between the SNPs in the 200-kb interval surrounding the leading SNP was evaluated.
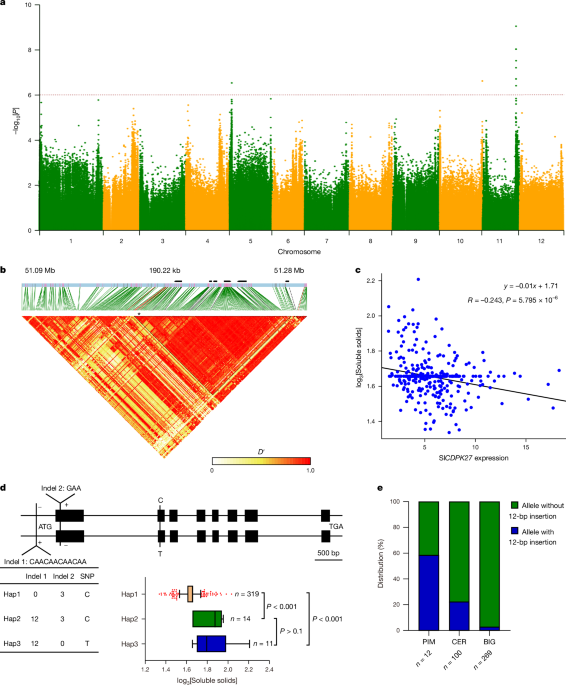
For association analysis of SlCDPK27 PCR amplification data with SSC, a total of 65 variants were generated. An SNP and 3-bp deletion, which lead to nonsynonymous mutation, and a 12-bp insertion, which could be recognized by RAV transcriptional repressor30,31, were retained for further analysis. Information on the variants is listed in Supplementary Table 2. For allelic variation analysis of SlCDPK27, a dataset of the three key variants was obtained through PCR amplification. The SNP, 12-bp insertion and 3-bp deletion were used to access haplotypes of SlCDPK27; only haplotypes with a total number of accessions of â¥3 were analysed. All primers used in this study are shown in Supplementary Table 9.
Transgenic functional validation
The single-guide RNAs for CRISPRâCas9 constructs were designed using the CRISPRdirect tool (http://crispr.dbcls.jp/). The CRISPRâCas9 binary vectors (pKSE402) were revised from the pKSE401 vector by replacing AtU6p with SlU6p (ref.â63). The recombinant pKSE402 vectors were designed to produce mutagenesis within the coding sequence of SlCDPK27 and SlCDPK26, using single-guide RNAs in combination with the Cas9 endonuclease gene (Fig. 2a and Extended Data Fig. 3a for the single-guide RNAs used in this study). Vectors with the correct insertion were introduced into Agrobacterium tumefaciens strain AGL1 competent cells, and tomato transformation was performed as described previously64. The transgenic lines were confirmed by PCR and sequencing. All experiments were performed using homozygous lines without T-DNA integration.
Physicochemical analysis
More than six red ripe fruits were collected from each line, and each fruit was measured for fruit weight and total SSC. The SSC was determined using a digital refractometer (PAL-1, ATAGO), adjusted and calibrated at 20â°C with distilled water and expressed as degrees Brix.
Content analysis of sugars
More than six red ripe fruits were collected from each line for sugar analysis. The mixed fruit pericarp was ground in liquid nitrogen, and then 200âmg of ground powder was diluted in 1.4âml of extraction buffer, with internal standard (8âmg arabinose). After sonication for 10âmin and centrifugation (13,000âr.p.m.) for 10âmin, the supernatants were filtered through a 0.22-μm polyethersulfone ultrafiltration membrane, twice, and then added to a solution of 100 μl extraction buffer, 895 μl acetonitrile and 5 μl 20% ammonia water for analysis. The content was measured by ultra-performance liquid chromatography with MS/MS (ACQUITY UPLC I-Class-Xevo TQ-S Micro, Waters). The detection was performed as described previously64.
For sugar analysis, an ACQUITY UPLC BEH Amide 1.7-μm column was used (2.1âÃâ100âmm; Waters). The mobile phase was composed of acetonitrile as solvent A, and 0.1% ammonia water as solvent B. The temperatures of the column and autosampler were 60â°C and 4â°C, respectively. Each sugar was separated by increasing solvent B from 10% to 20% in 2âmin, keeping at 20% for 6âmin, changing to 25% in 0.1âmin, keeping at 25% for 1.9âmin, then changing to 20% in 1âmin and keeping at 20% for 2âmin. The flow rate was 0.2âmlâminâ1. Data analysis was performed using MassLynx V4.1 (Waters).
RNA isolation and qRTâPCR
Total RNA was extracted from the fruit pericarp harvested at the ripening stages, using the RNA extraction kit (catalogue no. 0416-50, HUAYUEYANG Biotechnology), and the RNA was reverse transcribed, using GoScript Reverse Transcriptase (catalogue no. A5003; Promega), according to the manufacturerâs instructions. qPCR was performed using GoScript qPCR Master Mix (catalogue no. A6001; Promega) and the Bio-Rad CFX-96 real-time PCR with CFX Maestro 1.1 software (Bio-Rad). The relative expression levels of each gene were calculated using the 2âÎCt method. Three technical replicates were used to calculate the CT value, and three to five biological replicates were analysed. The tomato ACTIN gene (Solyc03g078400) was used as the internal reference.
Histochemical GUS staining
To examine the SlCDPK27 expression pattern by GUS staining, the 2,452-bp SlCDPK27 promoter region upstream of the ATG was amplified from genomic DNA. Then, the products were cloned into pENTR/D-TOPO to generate pENTR-SlCDPK27pro. SlCDPK27pro-GUS was generated by an LR reaction between pKGWFS7 and pENTR-SlCDPK27pro. SlCDPK27pro-GUS vector was then introduced into A.âtumefaciens strain AGL1 competent cells, and tomato transformation was performed as described previously64.
Different tomato tissues from the SlCDPK27pro-GUS transgenic lines were collected and incubated in GUS staining buffer containing 5-bromo-4-chloro-3-indolylb-d-glucuronide (X-gluc) as a substrate. Samples were incubated at 37â°C for 1âh. After incubation, the staining buffer was then changed to 70% ethanol for decolourizing.
Subcellular localization assay
To detect the subcellular localization of SlCDPK27 protein, full-length cDNAs of SlCDPK27 and SlCDPK27-CR1 were amplified from MM and the MM-CDPK27-CR1 mutant. The amplified fragments were cloned into pDONR/Zeo (Invitrogen) to generate pENTR-SlCDPK27 or pENTR-SlCDPK27-CR1, respectively. The SlCDPK27âGFP and SlCDPK27-CR1âGFP constructs were generated by LR reactions between pK7FWG2 and pENTR-SlCDPK27 or pENTR-SlCDPK27-CR1, respectively. Then, SlCDPK27âGFP and SlCDPK27-CR1âGFP were transformed into A.âtumefaciens strain GV3101, and the agrobacteria harbouring the constructs were infiltrated into N.âbenthamiana leaves. The plants were then grown in the dark for 24âh, followed by 48âh in a greenhouse under normal conditions. The transient GFP fluorescence in N. benthamiana leaf cells was observed under a Leica SP8 confocal microscope.
Sensory evaluation of sweetness
The sensory evaluation panel was organized twice (one in Shenzhen in March 2022, and the other in Beijing in July 2022). In this study, approximately 100 participants (aged 20â59 years) were selected for each of the sensory tests. These participants were required to be healthy and without any known oral diseases. The sensory test followed the Declaration of Helsinki, and the experimental protocol was approved by the Ethical Committee of Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences. All participants were informed about the sources of the genome-edited tomato materials and the sensory procedure beforehand, and signed informed consent forms before the sensory tests.
The two-alternative forced-choice test was performed to compare the difference in the sensory properties of the sweetness between fruits harvested from SlCDPK-knockout mutants and wild-type MM plants. A paired sample, consisting of a wild-type MM (control sample) and a SlCDPK-knockout mutant (test sample) fruit, was evaluated, according to ISO 5495:2005 (ref.â65). A total of three test samples were evaluated in this study, including MM-CDPK27-CR1, MM-CDPK27-CR2 and MM-CDPK27-CR2/MM-CDPK26-CR1 double-mutant plants. Each test sample was evaluated three times, and each time fruit was picked from three different plots. Each sample was placed in a transparent tasting cup labelled with random codes, and the paired samples were presented to the sensory assessors, for sensory evaluation, in a balanced order.
For each panel, about 100 volunteer assessors were asked to perform all of the tests. They were asked to evaluate pairs of samples and were required to indicate the sweeter sample of the paired tomato fruits. A 20â30âs break was provided between the different samples, and the assessors were requested to thoroughly rinse their mouths with purified water. Binomial distribution was used for the statistical analysis of paired comparison tests (ISO 5495:2005)65. If the number of correct responses was greater than or equal to the minimum number of correct responses required, at a specified significance level (in this study, αâ=â0.05), it can be concluded that the SlCDPK-knockout mutant sample has a higher sweetness than the wild-type MM sample. Otherwise, the difference was not significant. After the sensory evaluation, every six remaining fruits of each type were mixed into one sample for further glucose and fructose content analysis. Owing to COVID-19, we did not collect fruits for the sensory evaluation panel organized in Shenzhen in March 2022.
SlCDPK27 antibody production
A peptide containing 15 amino acids from the 6th to the 20th amino acids of SlCDPK27 (6GTPGNSSENKKKKNK20) was synthesized and used as an antigen to produce polyclonal antibodies in rabbits. The antibodies were purified by an antigenâspecific affinity approach by Shanghai Youke Biotechnology Co., Ltd.
Immunoprecipitation coupled with MS
For identification of the interaction proteins of SlCDPK27 by immunoprecipitation coupled with MS, fruits of MM at the red ripe stage were collected and ground in liquid nitrogen with an IKA A11 basic analytical mill (A11BS025). Total proteins were extracted with extraction buffer (300âmM Tris-HCl pHâ8.0, 600âmM NaCl, 4âmM MgCl2, 0.5% Triton X-100, complete EDTA-free protease inhibitor cocktail (Roche, cOmplete) and phosphatase inhibitor cocktail (Roche, PhosSTOP)). The extracts were centrifuged at 12,000âr.p.m. for 20âmin, and the supernatants were incubated with anti-SlCDPK27 (1:100 dilution) at 4â°C overnight, with the supernatant incubated with IgG used as the control, and then Pierce protein A/G magnetic beads (Thermo Fisher Scientific, 88802) were used to immunoprecipitate the SlCDPK27 protein. The immunocomplex was washed three times and resuspended with 100âµl extraction buffer. A 10âµl volume of sample was used for western blot analysis with SlCDPK27 antibodies (1:2,000 dilution), and horseradish peroxidase-conjugated goat anti-rabbit IgG (Hâ+âL) (ZSGB-BIO, catalogue no. ZB-2301, 1:10,000 dilution) was used as the secondary detection antibody. The remaining magnetic beads were separated on 10% SDSâPAGE gels, and then sent to PTM BIO Co., Ltd (Hangzhou, China) for analysis by liquid chromatography coupled with MS/MS.
Yeast two-hybrid assay
The yeast two-hybrid assays were performed using DUAL membrane starter kits (P01401-P01429, Shanghai OE Biotech) according to the manufacturerâs instructions. The ubiquitin moiety was split into two halves, and the N-terminal half with I13 substituted to glycine was used to prevent nonspecific binding to the C-terminal half of ubiquitin. The full-length cDNA of SlCDPK27 was cloned into the pBT3-STE bait vector to generate NubGâSlCDPK27 and the full-length cDNA of SlSUS3 was cloned into the pPR3-N prey vector to generate CubâSlSUS3, by gateway cloning. The fusion plasmids were co-transformed into yeast strain NMY51 and the yeast transformants were screened on SD/âLeu/âTrp and SD/âLeu/âTrp/âHis selective medium.
Firefly LUC complementation imaging assay
The full-length cDNAs of SlCDPK27, SlCDPK27-CR1, SlCDPK27-CR2 and SlCDPK26 were cloned into the pCAMBIA-nLUC-GW vector to generate SlCDPK27ânLUC, SlCDPK27-CR1ânLUC, SlCDPK27-CR2ânLUC and SlCDPK26ânLUC, respectively. Then the full-length cDNA of SlSUS3 was cloned into the pCAMBIA-cLUC-GW vector to generate cLUCâSlSUS3, by gateway cloning66. The plasmids were transformed into A.âtumefaciens strain GV3101. Different combinations shown in Figs. 5b,c and 6a,b were co-infiltrated into N.âbenthamiana leaves. The plants were placed in the dark for 24âh, followed by 48âh in a growth chamber under long-day conditions (16âh light and 8âh dark). Then, the infiltrated N.âbenthamiana leaves were sprayed with 1âmM luciferin, in 0.01% Triton X-100 solution, and kept in darkness for 5âmin to quench the fluorescence. A deep cooling CCD imaging apparatus (LB985 Night SHADE) was used to capture the fluorescence image in Fig. 5b, and the Tanon-5200 image system (Tanon, Shanghai, China) was used to capture the fluorescence images in Figs. 5c and 6a,b.
Recombinant protein production and purification
The full-length cDNAs of SlSUS3 were cloned into the pCold-TF vector to express HisâTF-tagged recombinant protein. The site-specific mutation in SlSUS3S11A was introduced by PCR, with the primers listed in Supplementary Table 9. The mutated fragments, confirmed by Sanger sequencing, were cloned into the pCold-TF vector to express HisâTF-tagged recombinant protein. The full-length cDNAs of SlCDPK27, SlCDPK27-CR1, SlCDPK26, SlSUS3 and SlSUS3S11A were cloned into the pET28GW vector to express His-tagged recombinant protein, by gateway cloning. The full-length cDNA of SlCDPK27-CR1 was also cloned into the pGEX-4T-1 vector to express GST-tagged recombinant protein for the in vitro kinase competition experiment. HisâSlCDPK27, HisâSlCDPK27-CR1, GSTâSlCDPK27-CR1, HisâSlCDPK26, HisâSlSUS3, HisâSUS3(S11A), HisâTFâSlSUS3, HisâTFâSlSUS3(S11A) and GST protein were expressed in Escherichia coli Rosetta (DE3) (catalogue no. EC1010, Shanghai Weidi Biotechnology), following induction with 1âmM isopropyl β–d-1-thiogalactopyranoside (IPTG) at 16â°C for 16âh, and then were purified using Ni-NTA resin (GE-Healthcare) and glutathione Sepharose 4B (GE-Healthcare) according to the manufacturerâs instructions.
In vitro phosphorylation assays
For the in vitro kinase assays, purified recombinant kinases (HisâSlCDPK27, HisâSlCDPK27-CR1 or HisâSlCDPK26) and substrate (HisâTFâSlSUS3 or HisâTFâSlSUS3(S11A)) were incubated at 30â°C in a kinase reaction buffer (50âmM Tris-HCl, pHâ7.5, 10âmM MgCl2, 1âmM dithiothreitol (DTT), 10âµCi [γ-32P]ATP) for 30âmin. The reactions were then stopped by adding 5ÃâSDS loading buffer (250âmM Tris-HCl, pHâ6.8, 10% (w/v) SDS, 0.5% bromophenol blue, 50âmM DTT and 50% glycerol) and boiled for 5âmin. The samples were then separated on 10% SDSâPAGE gels. After electrophoresis, the gels were stained with Coomassie brilliant blue as a loading control, and the phosphorylated proteins were visualized by autoradiography.
For the in vitro kinase competition experiment, GSTâSlCDPK27-CR1 and GST proteins with various concentration gradients were first incubated with HisâTFâSlSUS3 at 30â°C for 1âh. Then, HisâSlCDPK26 was added to the kinase reaction buffer for another 30âmin. The reactions were stopped by adding 5ÃâSDS loading buffer and boiled for 5âmin. The samples were separated on 10% SDSâPAGE gels and visualized by autoradiography.
Identification of phosphorylation sites of SlSUS3 by SlCDPK27
For analysis of phosphorylation sites of SlSUS3 in vitro, recombinant HisâSlCDPK27 and HisâTFâSlSUS3 proteins were incubated in kinase reaction buffer (50âmM Tris-HCl, pHâ7.5, 10âmM MgCl2, 1âmM DTT, 0.25âmM ATP) at 30â°C for 30âmin. Next, the proteins were separated on 10% SDSâPAGE gel, and the bands of HisâTFâSlSUS3 were cut out. Then, the HisâTFâSlSUS3 protein was digested with trypsin and subjected to analysis by liquid chromatography with MS/MS by the biological MS laboratory at the College of Biological Sciences at China Agricultural University.
Cell-free protein degradation assay
The cell-free protein degradation assay for SlSUS3 and SlSUS3(S11A) was performed as described previously67,68. Briefly, total proteins were extracted from full-red ripe fruits of wild-type, MM-CDPK27-CR1, MM-CDPK27-CR2, MM-CDPK26-CR1 and MM-CDPK27-CR2/MM-CDPK26-CR1 double mutant plants, in degradation buffer (300âmM Tris-HCl at pHâ8.0, 600âmM NaCl, 4âmM MgCl2 and 20âmM DTT). Recombinant HisâSlSUS3 and HisâSUS3(S11A) protein and 5âmM ATP were added to the extracts. The mixtures were incubated at room temperature (25â°C) for 1, 2 and 4âh. The reactions were stopped by the addition of SDS sample buffer. HisâSlSUS3, HisâSUS3(S11A) and actin proteins were detected by immunoblotting with anti-His (MBL, catalogue no. D291-3, 1:3,000 dilution) and anti-actin (Sigma, catalogue no. A0480, 1:10,000 dilution) antibodies, and horseradish peroxidase-conjugated goat anti-mouse IgG (Hâ+âL) (ZSGB-BIO, catalogue no. ZB-2305, 1:10,000 dilution) was used as the secondary detection antibody. Protein gel blot images were scanned, and the intensity of the images was quantified by ImageJ (National Institutes of Health).
Gas exchange and 13CO2 labelling of tomato
In vivo isotopic labelling with 13CO2 and flux estimation throughout leaf photosynthetic metabolism were performed as described previously69. A 30-l positive-pressure environmental chamber set at 28â°C, 50% humidity and around 200âμmolâmâ2âsâ1 light intensity was applied for labelling. After displacing CO2 with premixed gas with a N2/O2 ratio of 78/22, 4-week-old plants were labelled using premixed gas containing 13CO2 (Cambridge Isotope Laboratories) at a 13CO2/N2/O2 ratio of 0.33:78:21.967. Then, samples labelled for 0, 5, 10 and 20âmin were collected and immediately frozen with liquid nitrogen, and then stored at â80â°C for further analysis.
Analysis of metabolite labelling
A 100â±â3âmg quantity of ground powder of each sample was used for analysis of labelled metabolites. A 1.2âml volume of extraction buffer (dichloromethane/methanol 2:1) was added to 100â±â3âmg ground powder of each sample, and 300âμl water was added after vortexing five times. Then, 600âμl supernatant was transferred to a new tube and dried under nitrogen after centrifugation at 12,000g for 10âmin. After resuspension with 80âμl water, the samples were incubated in a refrigerator at 4â°C for 10âmin, and the supernatants were collected for analysis by liquid chromatography with MS after 10-min centrifugation twice at 12,000g to remove the precipitates.
A Dionex Ultimate 3000 UPLC system coupled with a TSQ Quantiva Ultra triple-quadrupole mass spectrometer (Thermo Fisher) equipped with a heated electrospray ionization probe was used for detection. Extracts were separated by a Synergi Hydro-RP column (2.0âÃâ100âmm, 2.5âμm, Phenomenex). A binary solvent system was used, with mobile phase A consisting of 10âmM tributylamine adjusted with 15âmM acetic acid in water, and mobile phase B consisting of pure methanol, using a 25-min gradient with mobile B gradually increased from 5% to 90%. Data were acquired in selected reaction monitoring mode for metabolites in positive-negative ion switching mode (Supplementary Table 10). The resolutions for Q1 and Q3 are both 0.7 full-width at half-maximum. The source voltage was 3,500âV for positive and 2,500âV for negative ion mode. The source parameters were as follows: capillary temperature, 350°C; heater temperature, 300â°C; sheath gas flow rate, 35; auxiliary gas flow rate, 10. Tracefinder 3.2 (Thermo) was used for metabolite identification and peak integration.
Genetic statistics and estimation of inbreeding
The variation map was constructed using previously published data7. Subsequently, the phylogeny was used to examine the population structure on the basis of the genome-wide SNPs using the IQTREE program (version 2.1.4)70. The 12-bp insertion and 1,406-bp deletion were combined to investigate the frequency of haplotypes of SSC11.1 and fw11.3 among PIM and BIG populations. To clarify the selective pattern of SSC11.1 (ch11_51.180â51.205âMb; SL2.50) and fw11.3 (ch11_55.250â55.290âMb; SL2.50), the independent phylogenies were constructed with the âGTRâ+âIâ+âGâ model using IQTREE70.
The genetic statistics were combined to examine the selection in the large region containing SSC11.1 and three fruit weight (fw) loci (ch11_48.0â56.3âMb; SL2.50). Tajimaâs D test compares the observed distribution of pairwise nucleotide differences to the expected distribution under a neutral model of evolution. A negative value suggests an excess of low-frequency mutations, which could be indicative of directional selection, population contraction or genetic hitchhiking71. The pairwise differentiation (FST), known as the fixation index, is a measure of genetic differentiation between populations. High FST values typically suggest high divergence between populations71. Tajimaâs D value was analysed by VCFtools (version 0.1.16), and the FST value was calculated using the Python script popgenWindows.py as described previously72,73. The recombination rate could be estimated on the basis of demographic history and the variation map, and SMC++ was used to infer the demographic history of BIG and PIM populations74. In addition, the variation map of PIM and BIG was integrated in Pyrho to evaluate the genome-wide recombination rate75.
Runs of homozygosity (ROHs) are extended stretches of homologous segments within genomes that reveal population history and trait architecture76. These regions indicate a lack of genetic variation and are often associated with inbreeding or recent ancestry. We performed the genome-wide ROH analysis on the basis of the variation map using the PLINK program (version 1.90b6.21)77 with the following parameters: –homozyg-kb 1,000 –homozyg-snp 10 –homozyg-window-het 3. Increased inbreeding levels are associated with both longer and more numerous ROHs. Furthermore, recent inbreeding events tend to result in longer ROH segments. To visualize these patterns, we used ggplot2 (version 3.4.4)78 to plot ROH length and number distributions across wild (PIM) and cultivated (BIG) populations.
Phylogenetic analysis of SlCDPK27 and SlCDPK26
Conserved domains and their corresponding Pfam-formatted HMM models (PF13499 and PF00069) were identified for SlCDPK26 and SlCDPK27 proteins by leveraging the InterPro database. Subsequently, genome sequence and annotation files encompassing a diverse array of 12 genomes were used for phylogenetic and sequence analyses, including a fern (Azolla filiculoides; https://fernbase.org/ftp/), a gymnosperm (G.âbiloba; https://ginkgo.zju.edu.cn/genome/ftp/; version-2021) and 10 angiosperm crops: S.âlycopersicum (https://solgenomics.sgn.cornell.edu/organism/Solanum_lycopersicum/genome; SL2.50), Solanum melongena (https://solgenomics.sgn.cornell.edu/organism/Solanum_melongena/genome; HQ-1315), Solanum tuberosum (https://genome.jgi.doe.gov/portal/; DMv6.1), Arabidopsis thaliana (https://genome.jgi.doe.gov/portal/; TAIR10), Capsicum annuum (http://www.pepperbase.site/node/3; CaT2T), Malus domestica (https://genome.jgi.doe.gov/portal/; v1.1), Manihot esculenta (https://genome.jgi.doe.gov/portal/; v8.1), Oryza sativa (https://genome.jgi.doe.gov/portal/; v7.0), Citrus sinensis (http://citrus.hzau.edu.cn/download.php; v3.0) and Citrullus lanatus (http://cucurbitgenomics.org/organism/21; 97103, v2). The names of protein annotations are assigned abbreviations derived from their scientific names for consistency. Homologous protein annotation was performed utilizing the hmmer program (version 3.4)79 and the previously identified Pfam-formatted HMM models. The annotated protein sequences were subjected to multiple sequence alignment using MAFFT (version 7.525)80 and phylogenetic tree construction using the neighbour-joining method in the MEGA program (version 11.0.10)81. Sequence alignment of CDPK proteins in clade III was visualized using the ggmsa (version 3.19)82 package in R.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.