Continuous directional rotation about an axis underpins macroscopic machinery and will probably prove crucial for future nanoscale machines. Synthetic molecular-scale rotary motors featuring light-driven rotation about a C=C double bond have been pioneered by the Feringa group over the past 25 years (refs. 9,10). Autonomous chemically fuelled directional rotation about a C–N single bond has been reported8,11,12,13,14, driven by opposing acylation and ester hydrolysis pathways that form a cyclic reaction network that is coupled to the exergonic hydrolysis of a carbodiimide fuel. Similar acylation–hydrolysis reaction networks have been used to achieve directional translational motion and to fuel out-of-equilibrium supramolecular assemblies5,6,15,16,17, but the lack of synthetic chemically fuelled molecular motors driven by alternative reaction networks reflects the challenge of finding and using suitable mutually compatible opposing reactions.
All life depends on redox chemistry, but despite the evolution in nature of several systems that allow reduction and oxidation pathways to run concurrently, cyclic redox reaction networks have not been used to drive unidirectional motion in artificial molecular motors. In this paper, we present a molecular motor in which autonomous unidirectional rotation about a C–C single bond is driven by a cyclic redox reaction network. Concurrent biocatalytic oxidation and chemical reduction reactions of a biphenyl motor create a cyclic reaction network that consumes simple fuels (oxygen and borane) to drive rotary motion about a C–C bond, with directionality governed by the enantioselectivity of the biocatalytic oxidation.
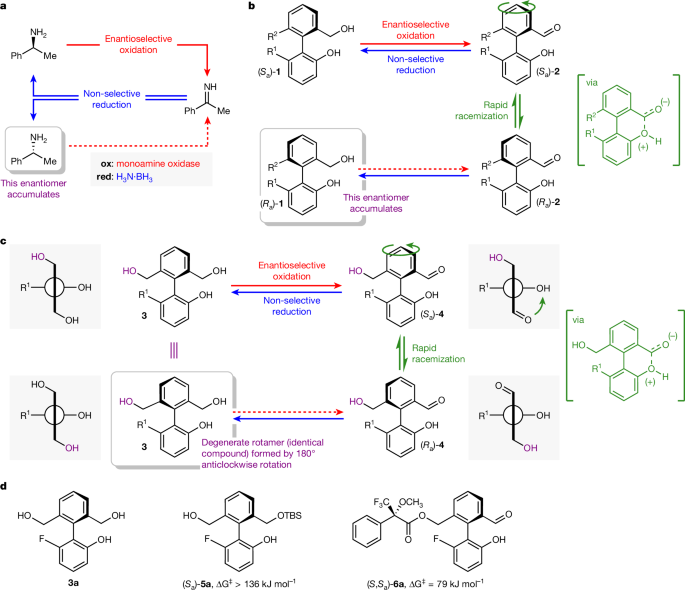
Our design builds on an archetypal cyclic reaction network in synthetic chemistry: cyclic deracemization18,19,20,21,22. Cyclic deracemizations enable the contra-thermodynamic enrichment of one enantiomer from a racemic mixture through the operation of a dissipative cyclic reaction network. For example, in the seminal redox-driven cyclic deracemization from Turner and co-workers shown in Fig. 1a (ref. 23), enantioselective oxidation of racemic α-methylbenzylamine is coupled with non-stereoselective reduction, leading to enrichment in the less reactive of the two amine enantiomers, (R)-α-methylbenzylamine. Concurrent oxidation and reduction is achieved by recourse to an oxidation biocatalyst (monoamine oxidase) that can function in the presence of a reducing agent, in this case, ammonia borane (H3N·BH3).
a, Cyclic deracemization of a chiral amine by way of an achiral imine, coupling enantioselective biocatalytic oxidation with non-selective reduction using ammonia borane23. b, Cyclic deracemization of an atropisomeric biphenyl by way of a pair of rapidly interconverting enantiomeric conformers; R1, R2 = unspecified substituents. c, Design for a unidirectional rotary molecular motor; R1 = unspecified substituent. d, Motor 3a and derivatives of 3a, (Sa)-5a and (S,Sa)-6a. Stereochemical assignment assumes that R1 has higher priority than OH and R2 has lower priority than CH2OH.
A related, but as yet underexplored, model for cyclic deracemization passes not through a transient achiral intermediate (the imine in Fig. 1a) but instead through a transient state that consists of a pair of rapidly interconverting enantiomers24. A model for such a deracemization is shown in Fig. 1b. Oxidation of the chiral atropisomeric diol 1 would give hydroxyaldehyde 2, which is expected to racemize rapidly at room temperature owing to a bonding interaction between the phenolic hydroxyl group and the aldehyde carbonyl (shown in square brackets). Covalent bonding interactions that lower energy barriers to bond rotation provide the basis for Bringmann’s ‘lactone’ method and other more recent strategies for the enantioselective synthesis of atropisomers25,26, and related covalent bonding interactions are exploited in the rotary motor of Leigh and co-workers and other stepwise rotary molecular motors8,27,28,29,30. Transient non-covalent bonding interactions31, as proposed for hydroxyaldehyde 2, have been used extensively in the asymmetric synthesis of atropisomers through dynamic kinetic resolution methods using organocatalysis, transition-metal catalysis and enzymatic catalysis26,32,33.
In analogy to Turner’s cyclic deracemization of a point chiral centre23 (Fig. 1a), deracemization of atropisomeric 1 occurs if the oxidation to hydroxyaldehyde 2 proceeds enantioselectively and is coupled to a non-selective reduction back to the diol 1. Such an atropisomeric deracemization is also accompanied by net directional motion: if the oxidation of 1 to 2 is enantioselective, the rapidly interconverting mixture of enantiomeric conformers of 2 is approached selectively from one direction, and every transformation of (Sa)-1 to (Ra)-1 results in a 180° anticlockwise rotation of the upper ring, as viewed from above, indicated by the curved arrow.
In Fig. 1c, we outline how one small change to the biphenyl deracemization substrate 1 reveals a simple design for a unidirectional rotary molecular motor. The same cyclic reaction network is applied to closely analogous molecule 3, which has two hydroxymethyl substituents in the ortho positions of the upper ring (Fig. 1c, in which R2 = CH2OH). Triol 3 is now achiral by virtue of the plane of symmetry that bisects the upper ring. Enantioselective oxidation desymmetrizes triol 3 to give an enantioenriched sample of chiral monoaldehyde (Sa)-4 that itself undergoes rapid racemization owing to the bonding interaction between the phenolic hydroxyl group and the aldehyde carbonyl (shown in square brackets). From monoaldehyde 4, the concurrent non-selective reduction does not return the enantiomer of the starting triol, because this achiral triol is, by definition, superimposable on its mirror image. Instead, it simply returns the starting material 3, ready to undergo another redox cycle. Crucially, however, 50% of those triol molecules 3 that return from a fully enantioselective oxidation–reduction cycle have undergone net 180° anticlockwise rotation of the upper ring during the racemization of the transient monoaldehyde intermediate 4. Thus, after one oxidation–reduction cycle, a proportion of triol 3 is returned in the form of a chemically indistinguishable (degenerate) rotamer in which the hydroxymethyl groups have exchanged positions by means of a 180° anticlockwise rotation. Oxidation of the second hydroxymethyl group (shown in purple) of 3 allows this rotated fraction of the starting material to enter the redox cycle a second time, and again a fraction will undergo 180° anticlockwise rotation. The dissipative cyclic redox reaction network thus no longer supports a deracemization; instead, it drives continuous autonomous net directional motion. We shall now describe how this design for a redox-driven molecular motor was reduced to practice.
The biphenyl rotary motor requires a symmetrically substituted phenyl ‘rotor’ ring with hydroxymethyl groups in each ortho position and a phenolic ‘stator’ ring substituted by R1 in the final ortho position. The nature of substituent R1 allows the rotational barriers of the reduced and oxidized states of the motor (triol 3 and monoaldehyde 4) to be adjusted for optimal function. We identified ortho-fluoro triol 3a (R1 = F), readily prepared through a five-step synthetic sequence, as a promising motor candidate with a configurationally stable reduced state 3a and a configurationally labile oxidized state 4a (Fig. 1d). Rotational barriers of 3a and 4a were estimated using desymmetrized derivatives34. An enantioenriched sample of silyl ether 5a showed no detectable racemization after two days at 100 °C in toluene (ΔG‡rot > 136 kJ mol−1; Supplementary Information Section 7.3). Acylation of 4a with Mosher’s acyl chloride 7 gave ester 6a (ref. 35), whose axial diastereoisomers show resolved signals by 1H and 19F nuclear magnetic resonance (NMR) spectroscopy. 19F NMR exchange spectroscopy analysis of the mixture of diastereoisomers of 6a indicates that the resolved signals undergo chemical exchange and reveals a barrier to rotation ΔG‡rot ≈ 79 kJ mol−1 at 40 °C in 2:1 DMSO:D2O (Supplementary Information Section 7.3).
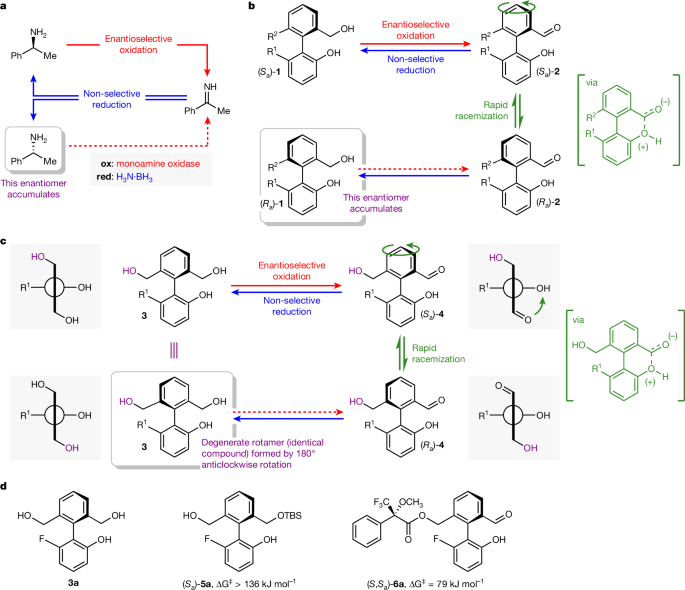
A cyclic reaction network that would allow concurrent enantioselective oxidation of achiral 3a and non-selective reduction of 4a was developed by using the cyclic deracemization of chiral analogue 1a as a model. Alcohol dehydrogenases (ADHs)36 have been used by Kroutil and co-workers in cyclic reaction networks leading to the deracemization of point-chiral alcohols37,38 and have also been used in the enantioselective synthesis of chiral biaryls through both desymmetrization and dynamic kinetic resolution processes39,40,41,42,43. Inspired by these studies, as well as the seminal report of concurrent biocatalytic oxidation and chemical reduction pathways in the deracemization of benzylic amines by Turner and co-workers23, we screened a library of ADHs using NADP as cofactor, an NADPH oxidase (YcnD) as the cofactor recycling enzyme44 and molecular oxygen as the terminal oxidant. Adding ten equivalents of ammonia borane at the outset of the ADH-catalysed oxidation revealed ADH 291 as an effective catalyst for the deracemization of 1a (Fig. 2). Optimization of the cofactor recycling system, pH and temperature provided (Ra)-1a in 96% yield and 94% enantiomeric excess (ee) after 24 h (Fig. 2a, entry 5; see Supplementary Information Section 5 for full optimization details). Monitoring the reaction mixture over time (Fig. 2b) established that the ee of 1a increased over about 24 h as 1a underwent repeated cycles of oxidation and reduction.
a, Optimization of deracemization of 1a: (±)-1a (10 mM), ADH 291, NADP, NADPH oxidase (YcnD (60 μM)), NaPi (100 mM), DMSO (10% v:v), H3N·BH3 (100 mM, 10 equiv.), O2 (air), ee analysis performed by high-performance liquid chromatography at 24 h. b, Yield and ee of 1a over time for conditions in entry 5; error bars are derived from triplicate reactions, in which the error given is the sample standard deviation between the triplicates.
Critically, the deracemization of 1a confirms the operation of the proposed redox cyclic chemical reaction network: enantiomeric enrichment can arise only through the continuous concurrent operation of the oxidation and the reduction pathways between 1a and 2a, which are not the microscopic reverse of each other. Also, this highly effective deracemization of 1a provides insight into the hierarchy of rates that characterizes the cyclic reaction network: for deracemization to occur, it is necessary that the interconversion of the enantiomeric conformers of 2a (that is, the enantiomerization of 2a) occurs faster than the reduction of 2a back to 1a. Both the reduction and enantiomerization of 2a must also occur faster than the oxidation of 1a. These kinetic constraints can be expressed as a hierarchy of rates, renant > rred > rox(Ra,Sa). Furthermore, the deracemization of 1a confirms that the oxidation is stereoselective, that is, rox(Sa)/rox(Ra) ≠ 1.
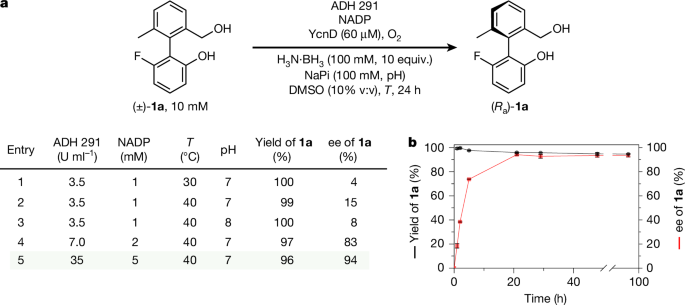
Having established an effective deracemization of 1a, we set about constructing an analogous cyclic redox reaction network under which motor candidate 3a would undergo rotary motion. We found ADH 291 to be an effective catalyst for the oxidation of triol 3a to monoaldehyde 4a. 3a was treated with ADH 291 under the conditions of the cyclic reaction network: addition of ten equivalents of ammonia borane at the outset of the reaction resulted in a reaction mixture comprising exclusively 3a after 48 h (Fig. 3a). When ammonia trideuteroborane (H3N·BD3) was used in place of ammonia borane, deuterium was incorporated at the benzylic positions of 3a (Fig. 3b; see Supplementary Information Section 8.3 for details), confirming that, under these conditions, chemically unchanged motor 3a is undergoing several cycles of oxidation and reduction.
a, Motor 3a under the conditions of the cyclic redox reaction network: 3a (10 mM), ADH 291 (35 U ml−1), NADP (5 mM, 50 mol%), YcnD (60 μM), H3N·BH3 (100 mM, 10 equiv.), O2 (air), NaPi (100 mM, pH 7), DMSO (10% v:v), 40 °C, 48 h. b, Ammonia trideuteroborane (H3N·BD3) in place of ammonia borane (H3N·BH3) in the cyclic redox reaction network leads to deuterium incorporation at the benzylic positions of 3a; error bars are derived from triplicate reactions, in which the error given is the population standard deviation between the triplicates. c, Conversion of 3a to all oxidation products (see Supplementary Information Section 8.4) over time during biocatalytic oxidation of 3a with the addition of intermittent pulses of H3N·BH3: 3a (10 mM), ADH 291 (35 U ml−1), NADP (5 mM, 50 mol%), YcnD (60 μM), NaPi (100 mM, pH 7), O2 (air), DMSO (10% v:v), 40 °C, pulses of H3N·BH3 (2 mM, 0.2 equiv.) added at 24, 48, 72 and 96 h (addition time points indicated with red arrows); error bars are derived from triplicate reactions, in which the error given is the population standard deviation between the triplicates.
The continued viability of the cyclic redox reaction network over extended periods of time was confirmed by subjecting 3a to sub-stoichiometric pulses of ammonia borane at regular intervals, rather than a large excess at the outset of the reaction (Fig. 3c; see Supplementary Information Section 8.4 for details). At the outset of the reaction, 3a was subjected to the standard oxidation conditions, and after 24 h, a combined yield of 45% of all oxidation products was observed. A pulse of ammonia borane (approximately 0.2 equivalents) was added at 24 h, and analysis after 1 h (16% yield of all oxidation products) and a further 24 h (51% yield of all oxidation products) confirmed that the oxidation system (ADH 291, NADP, YcnD and O2) was viable for at least 24 h. A further three pulses of ammonia borane (3× approximately 0.2 equivalents) were applied at 24-h intervals. After each pulse, the yield of all oxidation products returned to a similar value (approximately 50%), confirming the viability of the oxidation system over at least 96 h.
The experiments detailed in Fig. 3 confirm that 3a is a substrate for the cyclic redox reaction network, that 3a undergoes several sequential cycles of oxidation and reduction under the standard operating conditions (that is, [H3N·BD3]0 = 100 mM) and that the oxidation system remains viable over at least 96 h. Directional rotation of the motor also requires the rate of enantiomerization of 4a to be greater than the rate of its reduction (that is, renant > rred); if this condition is not met, the motor oscillates between the two redox states 3a and 4a but without accompanying rotation about the biaryl axis. Furthermore, effective motor operation requires that the rate of oxidation of 3a is the slowest of the three constituent steps (renant > rred > rox(Ra,Sa)) and that the oxidation of 3a proceeds stereoselectively, that is, rox(Sa)/rox(Ra) ≠ 1. For the deracemization of 1a, the emergence of enantiomeric enrichment proved that these kinetic constraints were met. However, directional rotation of 3a has no equivalent stereochemical consequence, so, to confirm that the motor is operating, we evaluated these rates independently.
To confirm the hierarchy of rates, renant > rred > rox(Ra,Sa), we determined each rate separately under conditions matching as closely as possible the operating conditions of the motor. The rate of enantiomerization, renant (that is, the rate of interconversion of (Ra)-4a and (Sa)-4a), was estimated from the barrier to rotation about the equivalent biaryl axis of the Mosher’s ester derivative (S)-6a in a mixture of D2O and DMSO: renant = 4.2 × 10−1·[4a] mM s−1 (Fig. 1d). The rate of the reduction of 4a, rred, was determined by monitoring the reduction of 4a to 3a in a NaPi (100 mM, pH 7)/DMSO (10% v:v) mixture at 313 K using ultraviolet–visible spectroscopy (see Supplementary Information Section 7.2 for full details) to give rred = 2.1 × 10−3·[H3N·BH3]·[4a] mM s−1. The undetectably low concentration of 4a at the steady state of the cyclic redox reaction network (rred ≫ rox(Ra,Sa); see below) precludes the determination of absolute values of renant and rred. However, rotary motor operation is contingent only on the relative rates of renant and rred and under the standard operating conditions (that is, [H3N·BH3]0 = 100 mM), rred/renant = 0.50 (<1, as required; see Supplementary Information Section 7 for details). The rate of oxidation of 3a (rox(Ra,Sa)) was determined by monitoring the oxidation of 3a to 4a under the operating conditions of the motor using high-performance liquid chromatography. In the initial stages of the oxidation reaction, [3a] and [O2] are constant and [3a] ≫ [ADH]. The resulting pseudo-zero-order plot gives rox(Ra,Sa) = 5.59 × 10−4 mM s−1. Without absolute values for renant or rred, we cannot compare them with this value numerically, but we make the assumption that rred ≫ rox(Ra,Sa), because 3a is the resting state of the cyclic redox reaction network. The pseudo-zero-order reaction kinetics noted in the determination of rox(Ra,Sa) remain valid during the operation of the cyclic redox reaction network because the concentration of 3a remains constant, being rapidly replenished by the excess of ammonia borane. Under the standard operating conditions of the motor, the rates of the three constituent processes that underpin rotary motor operation do thus indeed conform to the required hierarchy renant > rred > rox(Ra,Sa).
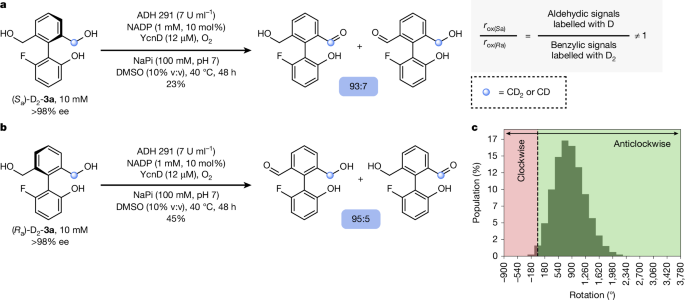
The final proof that 3a undergoes continuous directional rotation under the conditions of the cyclic redox reaction network requires evidence that the oxidation of 3a proceeds stereoselectively, that is, that rox(Sa)/rox(Ra) ≠ 1. This information cannot be provided by direct observation of enantiomeric enrichment in either starting material or product, because 3a is achiral and (Ra)-4a and (Sa)-4a racemize too fast for analysis of the enantiomeric ratio. Enantioselectivity can nonetheless be deduced from oxidations of enantiopure isotopomers (Sa)-D2–3a and (Ra)-D2–3a, which were made with 95% deuterium incorporation and known absolute configuration by methods described in Supplementary Information Section 2.6. As depicted in Fig. 4a, oxidation of (Sa)-D2–3a generates monoaldehyde 4a, in which the deuterium label (CD2 or CD) is distributed between the aldehydic CDO and benzyl alcoholic CD2OH positions in a ratio of 93:7 (1H NMR spectroscopy). Conducting the experiment with the isotopomer (Ra)-D2–3a gives a ratio of deuterium incorporation at the aldehydic and benzylic alcohol positions of 5:95. The enantioselectivity of the oxidation, rox(Sa)/rox(Ra), determines the ratio of deuterium incorporation (for example, for (Sa)-D2–3a, rox(Sa)/rox(Ra) = aldehydic signals labelled with D/benzylic alcohol signals labelled with D2), for which the difference between the ratios and the conversions observed for the two isotopomers arises from the kinetic isotope effect in the oxidation. Together, these experiments indicate that oxidation of unlabelled motor 3a to monoaldehyde 4a proceeds with an ee that falls in the range 85.7 ± 6.1% ee to 89.5 ± 2.7% ee, confirming that rox(Sa)/rox(Ra) ≠ 1. This experiment provides the first direct evidence of directional motion in an autonomously operating single-bond rotary motor.
a, Oxidation of (Sa)-D2–3a ((Sa)-D2–3a (10 mM, >98% ee), ADH 291 (7 U ml−1), NADP (1 mM, 10 mol%), YcnD (12 μM), O2 (air), NaPi (100 mM, pH 7), DMSO (10% v:v), 40 °C, 48 h), analysis of deuterium distribution at aldehydic CDO and benzylic CD2OH positions and example determination of rox(Sa)/rox(Ra). b, Oxidation of (Ra)-D2–3a ((Ra)-D2–3a (10 mM, >98% ee), ADH 291 (7 U ml−1), NADP (1 mM, 10 mol%), YcnD (12 μM), O2 (air), NaPi (100 mM, pH 7), DMSO (10% v:v), 40 °C, 48 h) and analysis of deuterium distribution at benzylic CD2OH and aldehydic CDO positions. c, Histogram illustrating the distribution of the net angles of rotation of 107 simulated molecules of 3a after 48 h of operation (see Supplementary Information Section 11 for details).
The experiments detailed in Fig. 3, the determination of a hierarchy of rates, and this direct observation of enantioselectivity in one of the constituent steps of the cyclic redox reaction network confirm that, under the standard reaction conditions, biphenyl 3a undergoes continuous net directional rotation about the C–C single bond. With the oxidation of 3a to 4a proceeding at a rate of rox(Ra,Sa) = 5.59 × 10−4 mM s−1 and assuming an average enantioselectivity of 87.6% ee, we determine that, for a statistically relevant population of motor 3a, the mean number of 360° rotations after 48 h of operation will be 2.35 (Fig. 4c; see Supplementary Information Sections 10 and 11 for details).
The motor is driven by the oxidation of the readily available commercial fuel ammonia borane, which—under the current conditions of motor operation—is accompanied by a high background fuel-to-waste reaction (see Supplementary Information Section 8 for details). Future work will aim to explore the fuel efficiency of the motor and to increase its rotational frequency by optimizing the rate and/or enantioselectivity of the oxidation, by varying the reductant and by developing a cyclic redox reaction network comprising stereoselective oxidation and reduction pathways37,45.
In summary, rotary motor 3a undergoes autonomous directional rotary motion about a C–C single bond, powered by a biocatalytic cyclic redox reaction network. Our studies confirm 3a as a substrate for the cyclic redox reaction network; a kinetic analysis establishes that the system conforms to a hierarchy of rates required for rotary rather than oscillatory motion; deuterium isotopomer studies confirm the stereoselectivity of the biocatalytic oxidation of 3a and allow, for the first time, the directionality of rotary motion of an autonomously operating single-bond molecular motor to be confirmed. Through this report of the first redox-driven single-bond rotary motor, biocatalysis emerges as a powerful tool for the design and development of autonomously operating chemically fuelled molecular motors.