Mouse experiments
Mouse experiments were performed in accordance with US Office of Laboratory Animal Welfare regulations and were approved by the Memorial Sloan Kettering Cancer Center Institutional Animal Care and Use Committee (IACUC protocol number 01-03-007). Mice were housed in solid-bottom, polysulfone, individually ventilated cages (IVCs) (Thoren Caging Systems) on autoclaved aspen-chip bedding (PWI Industries); γ-irradiated feed (LabDiet 50531, PMI) and acidified reverse osmosis water (pH 2.5 to 2.8) provided ad libitum. The cages also contained Nestlets, EnviroDri and/or EnviroPaks as environmental enrichment. The IVC system was ventilated at approximately 30 air changes hourly. HEPA-filtered room air was supplied to each cage and the rack effluent was exhausted directly into the building’s exhaust system. Cages were changed weekly in either a HEPA-filtered vertical flow change station or a class 2 type A biological safety cabinet. The animal holding room was maintained at 21.5 ± 1 °C, relative humidity between 30% and 70%, and a 12:12 h light:dark photoperiod. Mice were euthanized by CO2 asphyxiation for tissue collection. Male germline-specific Mre11-cKO mice were generated by crossing Mre11-flox (ref. 44) and Ngn3-cre (ref. 45) alleles as described elsewhere35. No randomization or blinding was performed.
Recombinant protein expression and purification
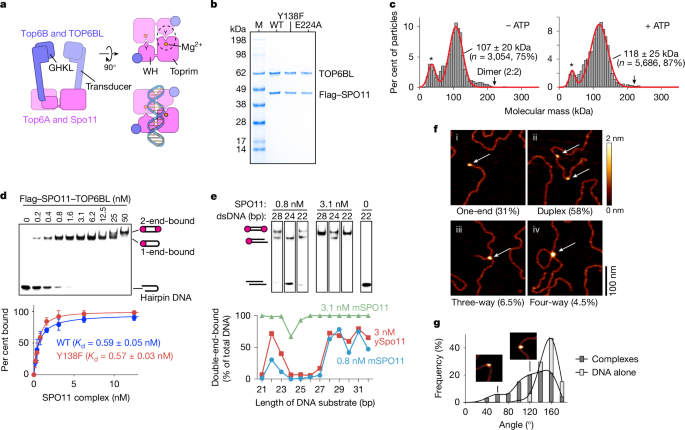
The full-length optimized coding DNA for mouse SPO11 (Uniprot: Q9WTK8-1) with a Flag tag followed by a TEV cleavage site at the amino terminus, and untagged TOP6BL (Uniprot: J3QMY9-1) was cloned into the pCDNA3.1(+) vector (Invitrogen). The SPO11 construct encodes for the β splicing isoform, which includes exon 2 that is skipped in the α isoform. SPO11β is both necessary and sufficient for most DSB formation in vivo, and the exon 2-encoded sequence is required for robust interaction with TOP6BL9,19,46,47,48. Although we cannot exclude that the Flag tag affects function, we note that even larger tags (FKBP or FRB) do not appear to interfere with activity and that the N-terminus of SPO11 is predicted to be unstructured in the AlphaFold 3 models. Point mutants (Y138F and E224A) were generated using QuikChange mutagenesis. Codon-optimized sequences encoding human FKBP1A (Uniprot: P62942), the FRB domain of human MTOR (Uniprot: P42345), and a glycine-serine (GS) linker were synthesized as gBlocks. These sequences were then cloned into the same vector as mouse SPO11, positioned between the Flag tag and the TEV cleavage site. Amino acid sequences of Flag-tagged wild-type SPO11 and the FKBP and FRB fusions are provided in Supplementary File 2.
FreeStyle 293-F cells (Invitrogen) were cultured in FreeStyle 293 expression medium (Gibco) under 5% CO2 in a Multitron-Pro shaker (Infors, 120 rpm) at 37 °C. The cell line was not authenticated or tested for mycoplasma. When the cell density reached 1.2 × 106 cells per ml, the plasmids were co-transfected as follows. For a 1 l cell culture, 1 mg of plasmids were pre-incubated with 2 mg of 40-kDa linear polyethylenimine (PEI) (Polysciences) in 25 ml of Opti-MEM (Gibco) for 25–30 min. Transfection was initiated by adding the mixture to the cell culture. About 16 h after transfection, sodium butyrate was added to a final concentration of 10 mM. Transfected cells were cultured for an additional 32 h (for a total of 48 h) before collection.
For each batch of protein purification, 1 l of transfected cells was collected by centrifugation at 3,100g and resuspended in lysis buffer containing 25 mM HEPES-NaOH, pH 7.5, and 500 mM NaCl. The suspension was supplemented with a protease inhibitor cocktail, including 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1× Halt Protease Inhibitor Cocktail (Thermo Scientific). Cells were lysed by sonication and centrifuged at 46,000g for 60 min. The supernatant was applied to anti-Flag M2 affinity gel (Sigma, A2220) and allowed to flow through by gravity at 4 °C. The resin was rinsed three times with lysis buffer. Proteins were eluted with lysis buffer containing 200 μg ml−1 Flag peptide (Sigma). The eluent was then concentrated using a 10-kDa cut-off Centricon (Millipore) and further purified by SEC (Superdex 200 Increase 10/300 GL, GE Healthcare) using UNICORN 7.6 (build 7.6.0.1306). Peak fractions were pooled and concentrated to 40 μl at a concentration of approximately 1.5 mg ml−1. Aliquots were frozen in liquid nitrogen and stored at –80 °C. Protein concentration was determined by absorbance at 280 nm using NanoDrop 8000 (version 2.3.3).
Oligonucleotide substrates for DNA binding and cleavage assays
Oligonucleotide sequences are provided in Supplementary Table 1. Hairpin substrates with a two-nucleotide 5′-overhang end for EMSAs were assembled by self-annealing oligonucleotides that were 5′-end labelled with [γ-32P]ATP (Perkin Elmer) and T4 polynucleotide kinase (NEB), followed by purification by native PAGE. Substrates for double-end binding experiments were generated by annealing complementary oligos to create 2-nt 5′ TA overhangs at both ends. The oligos were mixed in equimolar concentrations (10 mM) in STE buffer (100 mM NaCl, 10 mM Tris-HCl pH 8, 1 mM EDTA), heated, and slowly cooled. The substrates were then 5′-end labelled with [γ-32P]ATP (Perkin Elmer) and T4 polynucleotide kinase and purified by native PAGE.
The sequence of the oligonucleotide substrate for DNA cleavage was designed with one of the preferred cutting sites from pUC19 (Fig. 4a,b) placed in the middle of a 44 bp duplex flanked by A4 ssDNA loops (Fig. 4f). The 96-nt self-complementary oligonucleotide was first purified using a 15% polyacrylamide-urea gel (Invitrogen) and then labelled with [γ-32P]ATP (Perkin Elmer) and T4 polynucleotide kinase. The purified oligonucleotide was then annealed by heating and slow cooling. T4 DNA ligase was then used to seal the DNA nick, and the circular ligated oligonucleotide was purified from linear unligated molecules using another 15% polyacrylamide-urea gel. The circular oligonucleotide was then reannealed by heating and slow cooling.
Electrophoretic mobility shift assays
For hairpin substrates, binding reactions (10 μl) were carried out in 25 mM Tris-HCl pH 7.5, 7.5% glycerol, 100 mM NaCl, 2 mM dithiothreitol (DTT), 5 mM MgCl2, and 1 mg ml−1 bovine serum albumin (BSA) with 0.1 nM DNA and the indicated concentration of SPO11 complexes. Reactions were assembled on ice then incubated for 30 min at 30 °C and separated on a 6% DNA retardation gel (Invitrogen) at 100 V for 30 min. For binding to the oligonucleotide cleavage substrate, SPO11 complex was incubated with 0.1 nM DNA at 4 °C for 30 min in a buffer containing 25 mM HEPES-NaOH pH 7.5, 50 mM NaCl, 5 mM MgCl2, 7.5% glycerol, 1 mg ml−1 BSA, and 2 mM DTT. A 6% DNA retardation gel was pre-run at 100 V for 20 min with 0.5× Tris-borate supplemented with 5 mM MgCl2 as the running buffer, then the binding reactions were loaded and separated at 100 V for 40 min. For both substrates, gels were dried, exposed to autoradiography plates, and visualized by phosphor imaging using Amersham typhoon control software (version 4.0.0.4). Quantification was performed using GelBandFitter (version 1.7)49, and apparent Kd values were calculated by nonlinear regression in GraphPad Prism 10 (version 10.3.0 (461) for Mac OS X).
Atomic force microscopy
Linear plasmids for AFM were prepared by treatment of pUC19 with NdeI. SPO11–TOP6BL complexes were diluted to a final concentration of 4 nM in the presence of 1 nM (molecules) DNA in 25 mM HEPES-NaOH pH 6.8, 5 mM MgCl2, 50 mM NaCl, 10% glycerol. Mixtures were incubated at 30 °C for 30 min. A volume of 40 μl of the DNA-protein mixture was deposited onto pretreated mica with 3-aminoproply-trietoxy silane (APTES) (Pelco Mica Disc, V1, Ted Pella) for 5 min. The sample-deposited mica was rinsed with 1 ml molecular biology grade deionized water and dried gently with a stream of nitrogen. AFM images were captured using an Nanowizard V (JPK Scanning Probe Microscope Control Program 8.0.59.1) in AC Mode Imaging at room temperature. AFM probe (RFESPA-75, Bruker) with nominal frequencies of approximately 75 kHz and nominal spring constant of 3 N m−1 was used for imaging. Images were collected at a 2 Hz line rate with an image size of 2 × 2 μm at 512 × 512-pixel resolution. For data processing, images were exported with 3080-pixel resolution. The images were processed with JPK Data Processing software (version 8.0.59.1). ImageJ (version 1.54g) was used to quantify DNA bending angles.
Mass photometry
Mass photometry experiments were conducted using a Refeyn TwoMP instrument. A ready-to-use 6-well sample cassette (Refeyn) was placed at the centre of a clean, ready-to-use sample carrier slide (Refeyn), with each well designated for a single measurement. To find the focus, 15 μl of fresh MP buffer was loaded into the well. For SPO11 complexes, the buffer contained 25 mM HEPES-NaOH (pH 7.5), 150 mM NaCl, and 2 mM DTT. For complexes containing SPO11 fused to FKBP and FRB, the buffer was prepared with or without 5 μM rapamycin. The focal position was identified and maintained throughout the measurement using an autofocus system based on total internal reflection. Purified SPO11 or an equimolar mixture (1:1) of FKBP–SPO11 and FRB–SPO11 complexes was diluted to a concentration of 240 nM in MP buffer. Subsequently, 1 μl of the diluted SPO11 or FKBP–SPO11 plus FRB–SPO11 complex was added to the buffer drop, resulting in a final protein concentration of 15 nM. After autofocus stabilization, movies were recorded for a duration of 60 s. Data acquisition was performed using Refeyn AcquireMP (version 2024.1.1.0) and data analysis was carried out with Refeyn DiscoverMP (version 2024.1.0.0). Contrast-to-mass calibration was performed using a BSA protein standard (Sigma), containing BSA monomers and dimers with molecular masses of 66.5 and 132 kDa. Statistical analysis was performed using DiscoverMP, where the distribution peaks were fitted with Gaussian functions to obtain the average molecular mass of each distribution component. Plotting was carried out using GraphPad Prism 10.
DNA cleavage assays
Positively supercoiled pUC19 was prepared by incubating negatively supercoiled pUC19 (Thermo Scientific) with reverse gyrase (gift from S. Bahng of the K. Marians laboratory), followed by purification using a QIAquick PCR purification kit (QIAGEN) and verification via chloroquine-containing agarose gel electrophoresis as described50. Relaxed covalently closed pUC19 was generated by incubating negatively supercoiled pUC19 with E. coli topoisomerase I (NEB) and purified using a QIAquick PCR purification kit (QIAGEN). Linearized substrates were prepared by double digestion of pUC19 with EcoRI and SspI, followed by agarose gel extraction using a QIAquick Gel Extraction Kit (QIAGEN). For quantification, the linearized substrates were labelled at both 5′ ends using [γ-32P]ATP (Perkin Elmer) and T4 polynucleotide kinase (NEB).
Typical plasmid reactions (70 μl) were carried out in a buffer containing 25 mM HEPES-NaOH pH 7.5, 1 mM DTT, 0.1 mg ml−1 BSA (Sigma), 1 mM MnCl2, and 4 ng µl−1 pUC19 DNA. The reaction buffer was prepared on ice, and the recombinant proteins (usually 75–100 nM) were then added on ice. Unless otherwise stated, the reactions were incubated at 37 °C for the specified times. At each indicated time point, 11 µl of the reaction mixture was removed and terminated with 3 μl of STOP solution (375 mM EDTA, 5% SDS) and 1 μl of proteinase K (Thermo Scientific, 20 mg ml−1), followed by incubation for 30 min at 50 °C.
The reaction products were then mixed with 6× DNA loading dye (NEB) and separated by electrophoresis on a 1.2% agarose gel (Lonza) in TAE buffer. Using a customized vertical agarose gel system (gel length 14.5 cm, CBS Scientific), separation was carried out for 90 min at 80 V. The gels were subsequently stained with SYBR Gold (Invitrogen) and imaged using a ChemiDoc MP imaging system (Bio-Rad Image Lab Touch Software (version 3.0.1.14)).
Gel images were quantified using Image Lab (version 6.1.0 build 7) and GelBandFitter. Cleavage activity is expressed as percent of substrate utilized—that is, the fraction of DNA molecules that had sustained at least one detectable nick or DSB. It is important to note that this fraction underestimates total strand cleavage when DNA molecules sustain multiple breaks. This is because we cannot distinguish whether a nicked molecule has been nicked once or multiple times, we cannot distinguish whether a molecule with a DSB has also been nicked, and we cannot reliably quantify the number of breaks within the smear that results from multiple DSBs (plus covalently closed topoisomers when present). For simplicity, substrate utilization was calculated as the percentage of nicked circles and full-length linear molecules (DSBs) relative to the total DNA in each lane, subtracting any nicked DNA present in control (no protein) reactions as background (method 1). When multiple DSBs were clearly present—that is, where clear smearing appeared on the gel at later time points—we quantified substrate utilization for those lanes instead as the disappearance of the supercoiled band compared to time zero (method 2). Details about which quantification method was used can be found in the source data files for each graph. For plotting purposes, substrate utilization time courses were fitted to two-phase association curves by nonlinear regression in Prism 10. These curves are only intended to aid in visualization of trends and should not be viewed as a theoretically valid way to estimate underlying rate parameters.
For oligonucleotide cleavage assays, the substrate shown schematically in Fig. 4f was generated by self-annealing and ligation of a 5′-32P-labelled ssDNA oligonucleotide containing the preferred cleavage sequence (blue arrows) from pUC19 shown in Fig. 4b. Cleavage reactions were performed in a buffer containing 25 mM HEPES-NaOH pH 7.5, 5 mM MnCl2 (or 5 mM MgCl2), 0.1 mg ml−1 BSA, 1 mM DTT, and 0.02% NP-40. Mixtures containing 10 nM SPO11 complexes and 0.5 nM radiolabelled oligonucleotide were assembled at 4 °C and then immediately transferred to 37 °C to initiate the reaction. At each specified time point, 5 µl of the reaction mixture was taken out and rapidly quenched with 1.2 µl of 375 mM EDTA. The mixture was then digested with proteinase K for 30 min at 50 °C. Samples were loaded onto a 15% polyacrylamide-urea gel in 1× TBE running buffer and electrophoresed at 180 V for 50 min. Gels were then dried, exposed to autoradiography plates, visualized by phosphor imaging, and analysed using GelBandFitter. For experiments mixing SPO11(Y138F) and SPO11(E224A), 5 nM of each mutant complex was used. For SPO11(Y138F) alone, 10 nM of the mutant complex were used.
Note that the nicked product in Fig. 4g runs more slowly than the marker (M-Nick) because of the residual amino acid(s) left after proteolysis. We would therefore expect the DSB product to also migrate more slowly than the marker (M-DSB) for the same reason, but it instead comigrated closely with the marker. It may be that proteinase K is more effective at removing SPO11 residues from a DSB end than from a nick, but we cannot exclude the alternative possibility that the cleavage is happening somewhere other than the preferred sequence that is cut when in intact pUC19.
Assays for covalent protein–DNA complexes
For the CsCl ultracentrifugation and immunoprecipitation assays, cleavage reactions (60 µl) were carried out in a buffer containing 25 mM HEPES-NaOH pH 7.5, 1 mM DTT, 0.1 mg ml−1 BSA, 5 mM MnCl2, and 4 ng µl−1 pUC19 DNA. The reaction buffer was prepared on ice, and the recombinant proteins (325 nM final concentration) were then added on ice. The reactions were either terminated immediately or incubated at 37 °C for 11 min. To terminate the reaction for the ultracentrifugation assay, 50 µl of the reaction mixture was combined with 83.3 µl of a stop solution to yield final concentrations of 30 mM EDTA, 0.5% Sarkosyl, 5 M guanidine HCl. To terminate the reaction for the immunoprecipitation assay, 50 µl of the reaction mixture was combined with 12.5 µl of a stop solution to yield final concentrations of 37.5 mM EDTA, 0.5% SDS.
An adaptation of the ICE (in vivo complex of enzymes) assay was used for the immunodetection of proteins covalently bound to DNA51. In a new 5 ml centrifuge tube, 2 ml of 150% (w/v) CsCl solution was added, then 2 ml of buffer containing 10 mM Tris-HCl pH 8.0, 0.1 mM EDTA, and 0.5% Sarkosyl was layered on top. The stopped reaction mixture (133.3 µl) was then layered on top, and buffer was added until the tubes were full. The tubes were sealed, placed in a TN-1865 ultracentrifuge rotor (Thermo Scientific), and centrifuged at 42,000 rpm (~157,000g) for 17.5 h at 24 °C in a Sorvall wX+ Ultra Series ultracentrifuge (Thermo Scientific). The resulting DNA pellets with covalently bound proteins were washed with 70% ethanol and dissolved in 1× TE buffer (10 mM Tris-HCl pH 8.0, 0.1 mM EDTA) for 2 h at room temperature. Each sample was mixed with one-third volume of 25 mM sodium phosphate pH 6.5 buffer, then applied to a 0.45-µm nitrocellulose membrane (Bio-Rad) using a slot-blot vacuum manifold (Bio-Rad). No-protein negative controls (DNA only and buffer only) were included, and recombinant SPO11 complex (~10 ng total protein) was applied to adjacent slots as a control for detection. SPO11 protein was immunodetected using an anti-Flag–HRP monoclonal antibody (mouse, 1:1,000, Sigma A8592), followed by incubation with ECL Prime western blotting detection reagent (Amersham) and detection using a ChemiDoc MP imaging system (Bio-Rad).
For immunoprecipitation, the stopped reaction mixture (prepared as in the preceding paragraph for the ICE assay) was mixed with 237.5 µl of binding buffer (25 mM HEPES-NaOH pH 7.5, 500 mM NaCl, 5 mM EDTA, 1% Triton X-100) and 50 µl of anti-Flag magnetic agarose (Pierce A36797), which had been pre-washed twice with binding buffer. The mixture was incubated at room temperature with gentle shaking at 1,000 rpm for 60 min. After incubation, the magnetic agarose was washed twice with wash buffer (25 mM HEPES-NaOH pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100), then 30 µl of 2× Laemmli sample buffer (Bio-Rad) and 1 µl of proteinase K (Thermo Scientific, 20 mg ml−1) were added, followed by incubation for 30 min at 50 °C. The products were purified using the QIAquick PCR purification kit (QIAGEN), mixed with 6× DNA loading dye (NEB), and separated by agarose gel electrophoresis as in the DNA cleavage assays. The gels were subsequently stained with SYBR Gold (Invitrogen) and imaged using a ChemiDoc MP imaging system (Bio-Rad).
To investigate 5′ or 3′ SPO11 attachment, 10 µg of pUC19 was cleaved with EcoRI and labelled at the 5′ ends using T4 polynucleotide kinase (NEB) with [γ-32P]ATP or at the 3′ ends using Klenow (NEB) with [α-32P]dATP and [α-32P]TTP. Following labelling, the substrates were further digested with SspI to produce single-end labelled fragments, which were subsequently purified by agarose gel electrophoresis and extraction using QIAquick Gel Extraction Kit (QIAGEN). Cleavage reactions were performed at a substrate concentration of 2.3 nM (0.87 ng µl−1) in the presence of 25 mM HEPES-NaOH pH 7.5, 1 mM DTT, 0.1 mg ml−1 BSA, and 5 mM MnCl2. The reaction buffer was prepared on ice, and recombinant proteins (325 nM) were added on ice. Reactions were incubated at 37 °C for 2 h. To terminate the reactions, 70 µl of the reaction mixture was combined with 17.5 µl of stop solution to achieve final concentrations of 37.5 mM EDTA and 0.5% SDS. A 43.75 µl aliquot (half) of the stopped reaction mixture was treated with 1 µl proteinase K and incubated at 50 °C for 1 h. Samples, with or without proteinase K treatment, were then mixed with an equal volume of 2× urea TBE loading buffer (Invitrogen) and heated at 85 °C for 5 min. Subsequently, 10 µl of each sample was loaded onto a prewarmed 6% TBE-urea gel (Invitrogen) at 60 °C and electrophoresed at 180 V for 30 min. Gels were dried, exposed to autoradiography plates, and visualized using phosphor imaging.
TDP2-seq
In vitro cleavage assays for sequencing were conducted in 70 µl reaction buffer containing 25 mM HEPES-NaOH pH 7.5, 1 mM DTT, 0.1 mg ml−1 BSA and 1 mM MnCl2. For cleavage of pUC19, reaction buffer was mixed with 4 ng µl−1 DNA (final concentration) on ice, then recombinant SPO11–TOP6BL complex (100 nM) was added and the mixture was incubated at 37 °C for 30 min followed by inactivation with 1 µl proteinase K solution (Thermo Scientific, 20 mg ml−1) and 30 min incubation at 50 °C. Bacterial and yeast genomic DNA was purified by extraction with phenol:chloroform:isoamyl alcohol (25:24:1; Thermo Fisher) and ethanol precipitation from E. coli DH5α cells (Takara) or exponentially growing S. cerevisiae cells of the S288C strain background. For genomic DNA cleavage, reaction buffer was mixed with 2.5 µg DNA on ice. Recombinant SPO11 (300 nM) was then added and the mixture was incubated at 37 °C for 60 min and inactivated as above.
Sequencing libraries were prepared using a modified version of the S1-sequencing protocol52,53,54,55. In brief, testes from 16-week old (replicate 1) or 5-week old (replicate 2) mice were dissociated, and cells were embedded in agarose plugs as described55. For mapping in vitro SPO11 cleavage sites, DNA digested by SPO11 in vitro was embedded in plugs together with wild type C57BL/6 J mouse testis cells (0.5–1 million cells per plug), whose DNA acted as carrier during library preparation. Two plugs were prepared for each experiment. Following proteinase K and RNase A treatment, plugs were equilibrated in TDP2 buffer according to the TopoGEN protocol (50 mM Tris-HCl pH 8, 0.15 M NaCl, 10 mM MgCl2, 0.5 mM DTT, 30 μg ml−1 BSA, 2 mM ATP) and incubated with purified human TDP2 protein (490 pmol per plug, TopoGEN) at 37 °C for 30 min for removal of covalently linked SPO11 from DNA ends. Subsequent steps (end fill-in with T4 DNA polymerase (NEB), ligation to biotinylated adaptors, DNA purification, DNA shearing, ligation to a second adaptor at the sheared end, and PCR amplification) were all performed as described55 with the following minor modifications: (1) to reduce the loss of very small DNA fragments from diffusion out of the plugs, the washing step after ligation of the first adaptor was reduced from overnight to 1 h at 4 °C for experiments with pUC19; (2) the NEBNext End Repair Module (NEB, E6050S) was used for repair of DNA ends after shearing. DNA libraries were sequenced on the Illumina NextSeq 1000 platform (paired-end, 50 bp) using Real Time Analysis (version 3.1 or version 4.1) at the Integrated Genomics Operation at Memorial Sloan Kettering Cancer Center. BCL files were converted to FASTQ files using DRAGEN suite (version 4.2.7). Sample size (n = 2) was chosen based on prior evaluation of reproducibility of sequencing maps55. No statistical methods were used to predetermine sample size.
Sequencing reads were mapped against the pUC19, E. coli (ASM584v2), S. cerevisiae (sacCer3) or mouse (mm10) reference sequence using modified versions of previously published custom pipelines54. In brief, reads were mapped using bowtie2 (version 2.5.3)56 with parameters -N 1 -X 1000. Uniquely mapped reads were extracted and assigned to the nucleotide immediately next to the biotinylated adaptor. Statistical analyses were performed using R versions 4.2.3 and 4.3.2. Telomeres (500 bp at the ends of each chromosome) and ribosomal DNA (chr. XII:459,400–460,900) in the S. cerevisiae genome were masked before downstream analyses. Mitochondrial DNA and the 2µ plasmid were also excluded. Raw and processed TDP2-seq data were deposited at the Gene Expression Omnibus (GEO) (accession number GSE275291). Mapping statistics are found in Supplementary Table 2.
The pUC19 maps in Fig. 4a were smoothed with 21-bp Hann filter to simplify the display. For Fig. 4d, preferred cleavage sites were identified within previously defined hotspots43 in maps from Mre11-cKO mice, in which DSBs remain unresected35. Note the different vertical scales for base composition and sequence logo in Fig. 4d compared to the in vitro data in Fig. 4c, indicating weaker bias for in vivo data. For Fig. 4e, sites matching the in vitro bias (GNATNC, n = 11,423) or the non-preferred reverse sequence (CNTANG, n = 9,018) were identified within meiotic DSB hotspots, and strand-specific sequencing maps from Mre11-cKO mice were averaged over each group of sites.
Peak calling and base composition analyses
Top- and bottom-strand peaks were separately called as nucleotide positions with >3,000, >10 and >15 RPM of strand-specific read counts in pUC19, E. coli, and S. cerevisiae TDP2-seq data, respectively. Positions with less read counts than those located at –1 and +1 positions on top and bottom strands, respectively, were not defined as peaks because those reads could be false-positively enriched due to incomplete fill-in of 5′ overhangs at DNA ends before adaptor ligation. Base compositions were calculated for the strand from which TDP2-seq read was sequenced—that is, bottom-strand peaks were analysed with reorientation so that the nucleotide immediately next to the biotinylated adaptor should be located at –1 position relative to dyad axis on top strand. Sequence logos were generated using ggseqlogo (version 0.2)57, with base composition biases corrected for the genome average G+C content. For S. cerevisiae Spo11 in vivo data, the local G+C content around mapped DSB sites (47.8%) was used instead because it differed substantially from the genome average (38.5%). Motif matches in Fig. 4e were identified using dreg from the European Molecular Biology Open Software Suite (EMBOSS version 6.6.0)58 with default parameters.
AlphaFold 3
The pre-DSB SPO11 dimer models were generated using the AlphaFold 3 online service (https://golgi.sandbox.google.com)5. The input comprised two full-length mouse SPO11 (Uniprot: Q9WTK8-1) and TOP6BL (Uniprot: J3QMY9-1) proteins, two complementary DNA sequences (Supplementary Table 1), and four magnesium ions. Multiple DNA sequences were used to evaluate dependence of the model on the DNA composition. The sequences were selected to represent different SPO11 cleavage sites in yeast genomic DNA, and they varied in length from 36 to 44 bp. Each session produced five top-ranked models. We selected one representative model for figure preparation; this model’s coordinates are provided in Supplementary File 1. Structural alignments, analysis, and figure generation were performed in Chimera (version 1.18)59 and ChimeraX (version 1.8)60.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.