Cells are poor storytellers. They live complex, eventful lives — dividing, migrating, responding to their environment. But by the time they reach the laboratory, whatever narrative they once carried is gone. Plucked from their native context and pinned beneath a microscope or cracked open to expose the genetic ledger inside, they reveal only their final state, not the path that led them there. For cell biologists, it’s like glimpsing a play’s finale and working backwards to deduce the plot.
Molecular barcodes reveal tumour lineages
For decades, researchers have strained to reconstruct cellular backstories through indirect means: static snapshots, fluorescent footprints and algorithmic inferences. But these are forensic efforts at best, post mortems of a vanished process.
A new generation of DNA-recording tools promises to change that. By outfitting cells with the molecular equivalent of flight recorders, scientists can now log, in real time, the signals that cells receive, the paths they travel and the decisions they make — all inscribed indelibly in genomic ink.
Early versions of these recording systems were crude, able to track branching lineages of cellular fate through cycles of division, but little else. With advances in gene editing and single-cell sequencing technologies, however, cells can now also record the cues they sense, the states they pass through and even the timing and intensity of signalling events and gene activity.
Researchers have used these tools to trace developmental decisions in mouse embryos, capture gene activity in animal brains and analyse gene expression in engineered bacteria as they pass through the gut. Yet these remain early demonstrations, and the research community is still focused on expanding its technical capabilities.
“The field is nascent,” says Randall Platt, a genome engineer at the Swiss Federal Institute of Technology (ETH Zurich) in Basel. “There are more examples of concepts than practical use cases that have led to biological or medical breakthroughs.”

Jay Shendure helped to kick-start the development of cell-recording devices as a graduate student in 2003.Credit: Ron Wurzer/AP Images for HHMI_(2015)
Even so, momentum is building. Proof-of-concept studies are pointing towards real-world potential. And, backed by fresh investments, the pace of innovation is only expected to accelerate. “Many threads have matured,” says Jay Shendure, a genome scientist at the University of Washington in Seattle. The goal now, he explains, is to “push the engineering to the next level”.
Once optimized and broadly adopted, DNA-based recording tools could transform how scientists study development, disease and cellular decision-making in real time. “If we can now engineer cells to record in ways that are predictable and programmable,” Shendure says, “that just opens up all kinds of frontiers.”
DNA diaries
The dream of writing data into life’s code dates at least to 2003, when Shendure was an MD–PhD student at Harvard Medical School (HMS) in Boston, Massachusetts. That year, he and his supervisor, geneticist George Church, filed a patent for what they called a “nucleic acid memory device”: a primitive recording system in bacteria that used ‘recombinase’ enzymes to flip segments of DNA back and forth, like a toggle switch.
It was an ambitious vision for molecular data storage — but one constrained by the technology of the time. Even if the concept could, in principle, log gene activity, the DNA-sequencing methods then available made interpreting the records slow and impractical. “It was too early,” says Church. It took another decade — and the advent of next-generation sequencing, which drastically sped up DNA reading and reduced the cost — before the first molecular recorders capable of capturing large amounts of biological information over sustained periods could be built.
NatureTech hub
Synthetic biologist Timothy Lu, then at the Massachusetts Institute of Technology (MIT) in Cambridge, led the charge, showing in 2014 how engineered bacteria could convert fleeting transcriptional signals into changes in DNA. By pairing this process with the expression of recombinase enzymes, Lu and his colleagues created permanent genetic barcodes1. Two years later, they extended the approach to record the identity of diverse chemical stimuli and the order in which they occur2.
But this strategy soon hit a wall. Every extra piece of memory — every transcriptional event, signalling cue or developmental decision — demanded its own distinct enzyme that could operate independently of the others. And there were simply too few known recombinases to expand memory capacity in any substantial way.
A new technology platform was needed — and it arrived in the form of CRISPR–Cas9 gene editing.
In 2016, just a few years after CRISPR was adapted for editing human DNA, Shendure harnessed the tool to induce serial mutations at defined genomic sites, enabling his team to record lineage information across successive rounds of cellular division3. Known as GESTALT (genome editing of synthetic target arrays for lineage tracing), the system comprised two components: a stretch of DNA engineered with multiple CRISPR targets; and the Cas9 machinery that introduced edits at those sites (see ‘Cellular recording devices’).
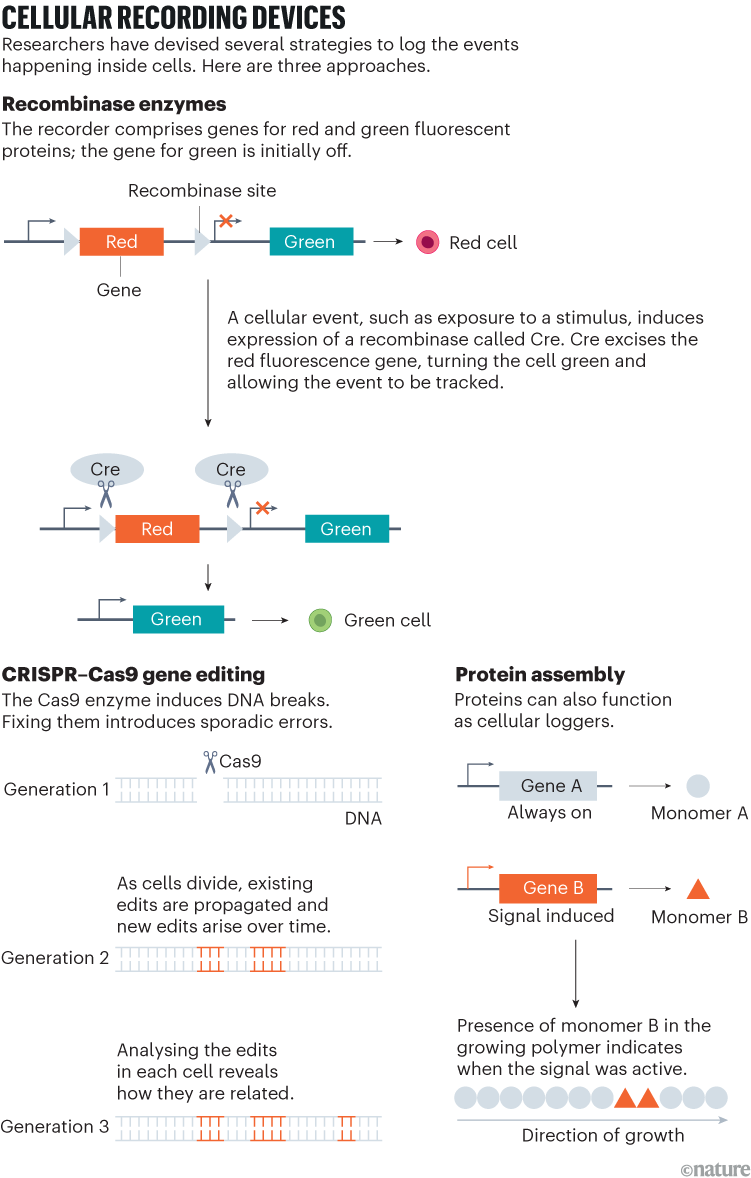
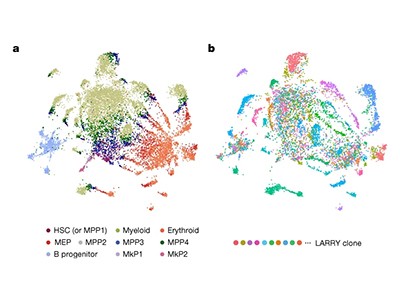
As cells divided, cuts were repaired imperfectly, leaving unique barcodes that accumulated over time. By sequencing these evolving barcodes, researchers could reconstruct cellular family trees across tissues or even entire organisms — as Shendure and his colleagues, including Aaron McKenna, then a graduate student and now a cancer geneticist at the Dartmouth Geisel School of Medicine in Lebanon, New Hampshire, demonstrated in cultured human cells and zebrafish embryos. Others soon followed suit with CRISPR-based recorders for use in mouse and cancer models, achieving much greater memory capacity than with recombinase systems, and opening fresh avenues for studying cell dynamics in development and disease.
Armed with these molecular timekeepers, researchers revealed hidden routes for how cancer cells spread (known as metastasis) as well as insights into how cancer clones expand4. Developmental biologists, using similar strategies in embryonic mice, challenged long-standing views of blood formation, uncovering progenitor cell populations that make lasting contributions to the blood system5. “The level of detail and resolution it supports is unparalleled,” Sarah Bowling, a developmental biologist at Stanford School of Medicine in California, says of the CRISPR-based lineage-tracing mice that she helped to pioneer.
But CRISPR–Cas9 also comes with trade-offs. Because it relies on double-strand breaks in DNA, it can be toxic to cells. And repeated cutting across target sites can erase the very record it is meant to preserve. As a result, although CRISPR enables richer lineage tracing than do older recombinase-powered tools, the barcodes it produces eventually saturate, cautions HMS synthetic biologist Sahand Hormoz. “If you delete enough, then everything’s deleted — and you don’t have any pattern you can use to reconstruct the lineage tree,” he says.
Prime time
That concern has pushed the field towards more-refined editing strategies such as base editors and, more recently, prime editors, that rewrite DNA without inducing double-strand breaks. Both are built on a CRISPR–Cas framework, but whereas base editors swap one DNA letter for another at a single position, prime editors use a guided reverse transcriptase to write short custom sequences into the genome.
These next-generation editors are pushing the field beyond lineage recording, allowing precise logging of gene expression and signalling dynamics, and empowering researchers to record not just that an event occurred, but also what preceded and followed it. “Before, there wasn’t really a good way to translate sensing what’s happening in the cell to genome editing,” says Junhong Choi, a synthetic biologist at the Memorial Sloan Kettering Cancer Center in New York City.
Patterns of DNA modifications provide a ‘barcode’ for cell-lineage tracing
Now, by tying the activity of prime editors to stimulus-responsive promoters or transcriptional circuits, researchers can convert transient molecular events — such as bursts of gene expression or protein–protein interactions — into permanent, time-stamped DNA changes. What’s more, this kind of genomic bookkeeping can be parallelized to respond to diverse cellular signals, creating simultaneous, chronological recordings of molecular activity in a type of multitrack data ledger, notes Theresa Loveless, a synthetic biologist at Rice University in Houston, Texas. “That modularity is a real advantage,” she says.
Collectively, these systems — accessible through the biological materials repository Addgene — represent more than just clever uses of genome editing. They are sophisticated pieces of synthetic-biology design that integrate finely tuned logic circuits with modular genetic components: sensors to detect cellular or environmental cues; actuators to relay inputs; and writers to inscribe information into DNA. “It wraps together many components of synthetic cell manipulation into these complex machines,” says Seth Shipman, a bioengineer at the Gladstone Institutes in San Francisco, California.
Still, even as these tools grow more powerful and intricate, researchers continue to find value in simpler approaches — including the kind that Shendure and Church first imagined more than two decades ago.
Last year, biophysicist Harry McNamara and his colleagues at Princeton University in New Jersey used a recombinase-based system to track the activity of key signalling molecules during early mouse-embryo development. This revealed distinct groups of cells whose molecular experiences were correlated with their eventual locations along the embryo’s anterior–posterior axis6. It was a task for which recombinases were well suited, because the biological question required only a durable yes/no record of signalling activity, not the detailed logs that CRISPR-based tools can provide, says McNamara, who is now at Yale University in New Haven, Connecticut.
“It allowed us to really look back in time and identify these key decision points when cells decide, in a sense, where they’re going to end up,” he says.
Fluorescent focus
Not everyone is sold on DNA as the universal recording medium, however.
Bioengineer Changyang Linghu at the University of Michigan in Ann Arbor, for one, favours a system in which protein monomers are added to a growing super-molecular assembly in response to transcriptional events7. This strategy works best in non-dividing cells such as neurons, because the protein assemblies are diluted during cell division. But the approach also offers a significant advantage: by pairing fluorescence imaging with event-specific protein tags, researchers can determine not just when and how strongly a gene was active, but also where in the cell this activity occurred.
Most DNA-based recording platforms lose this spatial context, because sequencing requires cell lysis, which destroys cellular architecture. To address this, synthetic biologist Amjad Askary at the University of California, Los Angeles, developed imaging-based systems that link fluorescence signals to genetic barcodes, enabling his team to trace transcriptional histories in intact tissues8. Notably, however, “these are nowhere close to as easy and adaptable for labs to do as single-cell sequencing”, says Askary.