Ethics
The laparotomy to obtain oocytes from X.âlaevis was carried out in accordance with the principles of the Basel Declaration and recommendations of Landratsamt Wuerzburg Veterinaeramt. The protocol under License number 70/14 from Landratsamt Wuerzburg, Veterinaeramt, was approved by the responsible veterinarian.
Molecular cloning
The pGEMHE vector51 was used for in vitro RNA synthesis and the following expression in X.âaevis oocytes. The binary pCAMBIA3300 vector with the UBQ10 promoter or the pCAMBIA1300 vector with the CaMV 35S promoter was used for Agrobacterium infiltration and stable transformation of plants.
Substitutions were introduced by QuikChange site-directed mutagenesis PCR. The pCambia1300 NES-2ÃR-GECO1 was cloned using the USER cloning technique52. For other constructs, all of the fragments with suitable restriction sites were introduced into the pGEMHE vector or the binary vectors pCAMBIA3300 with T4 DNA ligase (Thermo Fisher Scientific).
Plasmid extractions from Escherichia coli cultures were carried out using the MiniPrep 250 Kit (QIAGEN) according to the manufacturerâs instructions. All constructs were verified by sequencing (Eurofins Genomics). The sequences of XXM 1.1 and XXM 2.0 are available in the Supplementary Information.
Molecular engineering and development of XXM 2.0
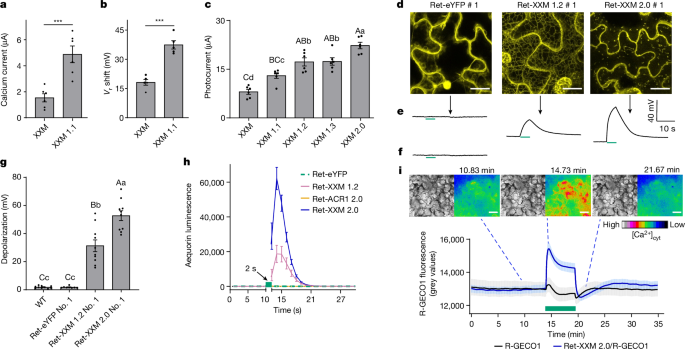
ChR2-XXM (D156H) was selected as a template to start molecular engineering owing to the high expression level and already enhanced Ca2+ permeability19. As no specific ion-selective filter is identified in ChR2, we anticipated that the three (extracellular, centre and intracellular) molecular gates (ECG, CG and ICG) might contribute to modulating ion-selective properties of ChR2, in addition to its gating function. Considering known substitution sites of ChR2 variants with modified ion selectivity in relation to the crystal structure indeed suggests a possibility to modulate ion selectivity using the ChR2 structure generated by PyMOL53 (Extended Data Fig. 1b). Within the three-dimensional structure, the internal cavities were calculated using HOLLOW, which is a published python script54. Some gating residues (H134 and E83 in ICG, and Q117 and R120 in ECG) and residues located in close proximity to the gate (E101 near ECG) were therefore substituted (Extended Data Fig. 1b). S63 (when combined with D156H) and E90 substitutions in CG have been reported to decrease Ca2+ conductance55,56, and therefore were not tested here. The resulting variants were first tested by two-electrode voltage-clamp analysis with X.âlaevis oocytes for comparison of photocurrent amplitude (Extended Data Fig. 1c). Variants exhibiting high photocurrent amplitude were next studied to determine the ion selectivity (reversal potential shift comparison) by changing extracellular ion concentrations (Extended Data Fig. 1d, see details in the following section) for selecting the superior candidate with high Ca2+ conductance (Fig. 1). To further enhance its membrane trafficking, N-terminal truncation of ChR2 and addition of signal peptides as recently reported4 were explored and examined by fluorescence imaging along with a comparison of photocurrents in X.âlaevis oocytes.
Two-electrode voltage-clamp analysis with X.
laevis oocytes
The AmpliCap-MaxT7 High Yield Message Maker Kit (Epicentre Biotechnologies) was used to synthesize XXM, XXM 1.1, XXM 1.2, XXM 1.3 and XXM 2.0 complementary RNA (cRNA). All of the cRNAs were stored in nuclease-free water at â20â°C. Oocytes were injected with 30âng cRNA and incubated in ND96 solution (96âmM NaCl, 2âmM KCl, 1âmM CaCl2, 1âmM MgCl2, 10âmM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4) at 16â°C for 2 days. Two-electrode voltage-clamp recordings were carried out at room temperature with a two-electrode voltage-clamp amplifier (TURBO TEC-03X, NPI Electronic). Electrode capillaries (diameter 1.5âmm, wall thickness 0.178âmm; Hilgenberg) were pulled by a vertical puller (PC-10, Narishige) and filled with 3âM KCl, with tip resistance of 0.4â1âMΩ. A USB-6221 DAQ interface (National Instruments) and WinWCP V5.3.4 software (University of Strathclyde, UK) were used for data acquisition. Blue light illumination was supplied by a 473-nm laser (Changchun New Industries Optoelectronics Tech).
Photocurrents of different XXM versions in response to blue light illumination (473ânm, 3âmWâmmâ2) were compared at a holding potential of â90âmV in a standard recording solution (96âmM NaCl, 2âmM KCl, 1âmM BaCl2, 1âmM MgCl2, 10âmM HEPES, pHâ7.6). For the reversal potential shift (mV) comparison, photocurrents of XXM and XXM 1.1 were recorded in both buffer A (119âmM N-methyl-d-glucamine, 0.8âmM BaCl2, 5âmM HEPES, pHâ7.6) and buffer B (80âmM BaCl2, 5âmM HEPES, pHâ7.6). Blue light (473ânm, 3âmWâmmâ2) was applied to activate different XXM variants. In the experiment for the comparison of calcium currents between XXM and XXM 1.1, 10âmM 1,2-bis(o-aminophenoxy)ethane-N,N,Nâ²Nâ²-tetraacetic acid (BAPTA) was injected to block the endogenous Ca2+-activated chloride channels of oocytes. The Ca2+ currents triggered by 3âmWâmmâ2 blue light were measured in the bath solution containing 80âmM CaCl2 and 10âmM 3-(cyclohexylamino)-2-hydroxy-1-propanesulfonic acid (CAPSO) at pHâ9 and a holding potential of â90 mV.
The action spectrum for XXM 2.0 was detected with light of different wavelengths. Light of different wavelengths (399.3ânm, 422ânm, 440.7ânm, 456ânm, 479.5ânm, 496ânm, 516ânm, 541ânm, 562ânm and 595ânm) was obtained by narrow bandwidth interference filters (Edmund Optics) together with a PhotoFluor II light source (89 North). Equal photon flux was set for each wavelength. Photocurrents were detected when treated with light of distinct wavelength and normalized to the maximal stationary currents triggered by blue light (456ânm). The light intensities were measured with a Plus 2 Power & Energy Meter (Laserpoint).
Agrobacterium transformation
The Agrobacterium tumefaciens strain GV3101 was collected by centrifugation after 1âday of culture and washed twice with sterile distilled water. All of the plasmids described for plant expression were transformed into A.âtumefaciens by an electroporation protocol57. Monoclonal colonies were selected from lysogeny broth (LB)âagar plates with 100âμgâmlâ1 kanamycin, 25âμgâmlâ1 gentamycin and 10âμgâmlâ1 rifampicin at 28â°C and cultured in LB medium with 100âμgâmlâ1 kanamycin, 25âμgâmlâ1 gentamycin and 10âμgâmlâ1 rifampicin at 28â°C. The colonies were confirmed by PCR.
Agrobacterium infiltration of N.
benthamiana leaves
For Agrobacterium infiltration, 30â37-day-old N.âbenthamiana plants grown in the greenhouse (40â60 kilolux light irradiation from 08:00â20:00, 24â26â°C) were used. Transient transformation of N.âbenthamiana plants was carried out according to the protocol of ref.â58. Briefly, agrobacteria were cultured in LB medium with 150âµM acetosyringone at 28â°C for about 16â18âh, collected by centrifugation and washed twice with infiltration buffer (10âmM MgCl2, 10âmM 2-morpholinoethanesulfonic acid (MES) (pH was adjusted to 5.6 by KOH), 150âμM acetosyringone). The final concentration was adjusted to 0.4 at an optical density of 600ânm (OD600nm) in infiltration buffer. A 1-ml syringe was used to infiltrate the resuspended agrobacteria into the leaves through the abaxial epidermis. The infiltrated plants were grown in 650ânm red light (light intensity of about 30âµWâmmâ2; cycles of 14âh light at 26â°C/10âh dark at 16â°C).
Stable transformation of N.
tabacum plants
N.âtabacum (N.âtabacum cultivar Petit Havana SR1) seeds sterilized by 6% NaOCl were germinated and grown in 500-ml sterile plastic boxes on agar plates (Murashige and Skoog medium including vitamins and MES (Duchefa Biochemie), 3% sucrose, 0.8% Gelzan (Sigma-Aldrich), pH 5.8 with KOH) in stable culture conditions of cycles of 14âh light at 26â°C/10âh dark at 16â°C. The A.âtumefaciens strain GV3101 harbouring the pCAMBIA3300 vector with BASTA resistance or the pCAMBIA1300 vector with hygromycin resistance was used for N.âtabacum transformation as described previously59 with some minor modifications. Agrobacteria were collected and washed twice with sterilized MS solution (Murashige and Skoog medium including vitamins and MES (Duchefa Biochemie), 3% sucrose, pHâ5.8 with KOH). The final concentration was adjusted to OD600nmâ=â0.1. Sterilized leaves were cut into pieces of about 2âcm2 and soaked in the resuspended agrobacteria solution for 20âmin. The wet leaf pieces were dried, placed on plant growth medium and transferred after 3 days to a callus-inducing medium (Murashige and Skoog medium including vitamins and MES (Duchefa Biochemie), 3% sucrose, 0.8% Gelzan (Sigma-Aldrich), pH 5.8 with KOH, 20âµgâmlâ1 dl-phosphinothricin (Duchefa Biochemie) or 30âmgâlâ1 hygromycin B (Thermo Fisher Scientific), 500âµgâmlâ1 ticarcillin disodium (Duchefa Biochemie), 100âmgâlâ1 myo-inositol, 1âmgâlâ1 thiamine hydrochloride, 1âmgâlâ1 6-benzylaminopurine and 100âµgâlâ1 1-naphthaleneacetic acid (Sigma-Aldrich)) and cultured under 650-nm light-emitting diode (LED) red light with light intensity of about 30âµWâmmâ2. The pieces of leaves, explants and calli were transferred to new callus-inducing medium every 2 weeks. Generated shoots were decapitated and moved onto rooting medium (Murashige and Skoog medium including vitamins and MES (Duchefa Biochemie), 3% sucrose, 0.8% Gelzan (Sigma-Aldrich), pH 5.8 with KOH, 20âµgâmlâ1 dl-phosphinothricin (Duchefa Biochemie) or 30âmgâlâ1 hygromycin B (Thermo Fisher Scientific), 500âµgâmlâ1 ticarcillin disodium (Duchefa Biochemie), 100âmgâlâ1 myo-inositol, 1âmgâlâ1 thiamine hydrochloride)) and, after root formation, were grown on soil in 650 nm red light (about 30âµWâmmâ2, cycles of 14âh light at 26â°C/10âh dark at 16â°C).
The transgenic plants were verified by eYFP fluorescence or R-GECO1 fluorescence in leaves. Seeds of individual plants were collected and selected for BASTA or hygromycin resistance using selection medium (Murashige and Skoog medium including vitamins and MES (Duchefa Biochemie), 3% sucrose, 0.8% Gelzan, pHâ5.8 with KOH, 20âµgâmlâ1 dl-phosphinothricin (Duchefa Biochemie) or 30âmgâlâ1 hygromycin B (Thermo Fisher Scientific)). Homozygous lines were used for experimental studies.
Confocal microscopy and image processing
A confocal laser scanning microscope (Leica SP5, Leica Microsystems CMS) controlled by Leica LAS AF (Version 2.7.3.9723, Leica Microsystems) was used to subcellularly localize the rhodopsinâeYFP fusions in plant cells or X.âlaevis oocytes. The yellow fluorescence was observed with a dipping 25à HCX IRAPO 925/0.95 objective in N.âbenthamiana leaves 3 days post Agrobacterium infiltration, in N.âtabacum leaves after 45 days grown in red light (light intensity of about 30âµWâmmâ2; cycles of 14âh light at 26â°C/10âh dark at 16â°C) and in X.âlaevis oocytes 2 days post injection. eYFP was excited at 496ânm and fluorescence was captured between 520 and 580ânm. N.âbenthamiana and N.âtabacum leaf discs were placed upside down for yellow fluorescence detection. FIJI IMAGEJ-win64 software60 was used for image processing.
Aequorin-based cytoplasmic free Ca2+ measurements
Co-infiltration of agrobacteria with 10âμM of coelenterazine (PJK Biotech) was carried out as described in the âAgrobacterium infiltration of N.âbenthamiana leavesâ section under red light conditions. After 2âdays in the red light growth room, aequorin luminescence from the infiltrated leaves was measured by a homemade luminometer. Luminescence was detected by a photomultiplier (Photo Counting Module MP 1983 RS CPM, Perkin Elmer) controlled by IGI-MPRS232 (IGIsystems). Labview 14.0.0 (National Instruments) was used to control the shutter and LEDs. A 520-nm green light LED (from WINGER, WEPGN3-S1) with a light intensity of 50âμWâmmâ2 was used to activate rhodopsins. To prevent the LED light from being detected by the photomultiplier, an additional shutter (Uniblitz, VCM-D1) was installed.
Live-cell imaging and all-optical physiology measurements
Live-cell imaging experiments were carried out using transiently transformed N.âbenthamiana leaves, transgenic N.âtabacum leaves (Ret-XXM 2.0 with R-GECO1, Ret-ACR1 2.0 with R-GECO1, R-GECO1, Ret-XXM 2.0 with pHuji and pHuji) or transiently transformed N.âbenthamiana mesophyll protoplasts (R-GECO1 and Ret-XXM 2.0 with R-GECO1). The A.âtumefaciens strain GV3101 harbouring the corresponding pCAMBIA vectors and A.âtumefaciens strain K19 were cultured at 28â°C overnight. The infiltration solution for co-expression of Ret-XXM 2.0 with R-GECO1 contained A.âtumefaciens strain K19 (OD600nmâ=â0.3) and A.âtumefaciens strain GV3101 harbouring the pCAMBIA plasmids (pCAMBIA3300 vector carrying Ret-XXM 2.0, pCAMBIA1300 vector carrying NES-2à R-GECO1; OD600nmâ=â0.4 for both). Control plants were infiltrated with infiltration solution containing A.âtumefaciens strain K19 (OD600nmâ=â0.3) and A.âtumefaciens strain GV3101 harbouring the pCAMBIA1300 vector carrying NES-2à R-GECO1 (OD600nmâ=â0.4). [Ca2+]cyt measurements were carried out 3 days post infiltration. Mesophyll protoplasts were prepared from the transiently transformed N.âbenthamiana leaves from which the abaxial epidermis was peeled off. The leaf pieces without the main vein were incubated in enzyme solution (1% BSA, 0.05% pectolyase Y23, 0.5% cellulase R-10, 0.5% macerozym R-10, 1âmM CaCl2, 10âmM MES, 500âmM d-sorbitol, pHâ5.6 with Tris) for 2âh. Following enzymatic digestion, cells were filtered through a 100-µm mesh. Protoplasts were collected by low-speed centrifugation (80g) without acceleration at 4â°C. Protoplasts were washed twice using precooled wash solution (1âmM CaCl2, 500âmM d-sorbitol, pHâ5.6 with Tris) and finally resuspended in precooled wash solution and stored on ice until use. Leaf disc samples with a diameter of 5âmm were prepared by peeling the abaxial epidermis off and gluing leaf discs upside down with medical adhesive (ULRICH Swiss) on custom-made recording chambers. The samples were allowed to recover in the dark in bath solution (1âmM KCl, 1âmM CaCl2, 10âmM MES, and 1,3-bis(tris(hydroxymethyl)methylamino)propane (BTP), pHâ 6.0) at room temperature (about 25â°C) overnight before R-GECO1 or pHuji fluorescence measurement and all-optical experiments were carried out.
The microscope setup to carry out live-cell imaging is described in detail elsewhere61. R-GECO125 and pHuji26 were excited with 570ânm excitation light. VisiView software (Version 2.1.1) was used to simultaneously control R-GECO1 imaging and triggering of local green light (532ânm, 180âμWâmmâ2) illumination by a solid-state laser (Changchun New Industries Optoelectronics Tech) or global green light (520ânm, 9âμWâmmâ2) illumination by a homemade LED device (LED from WINGER, WEPGN3-S1). XXM 2.0 activation by green light was carried out in the 5âs interval time during R-GECO1 or pHuji imaging. Green light illumination was terminated more than 1âs before R-GECO1 was excited to avoid photoswitching effects of R-GECO1 during optogenetic stimulation as reported previously62. A dichroic mirror (HC593 (F38-593), AHF Analysetechnik) combined with a high-speed filter wheel equipped with bandpass filters for R-GECO1 or pHuji (ET 624/20ânm) was used to detect the red fluorescence. During the detection of [Ca2+]cyt signal triggered by XXM 2.0 stimulation when a different light condition was used, a 2âs interval time was set during R-GECO1 illuminations. It should be noted that the light pulse protocols used to set defined [Ca2+]cyt signatures must be customized for each particular cell system or plant line and cannot act as a blueprint as features such as the expression level and cell type used or the Ca2+ homeostasis will probably influence the signature.
To avoid undesirable XXM 2.0 or ACR1 2.0 activations during bright-field imaging, the microscope white light source was covered by a primary red filter (Lee filter 106). Simultaneous plasma membrane potential and R-GECO1 fluorescence recordings were carried out in R-GECO1, Ret-XXM 2.0 with R-GECO1, Ret-ACR1 2.0 with R-GECO1 samples. Current-clamp-based voltage recordings were carried out by microelectrode impalement as described elsewhere61. Glass microelectrodes filled with 300âmM KCl were connected to the microelectrode amplifier (TEC-05X; NPI Electronic) equipped with head stages of more than 1013âΩ input impedance. The reference electrodes were filled with 300âmM KCl. A piezo-driven micromanipulator (Sensapex) was used to direct the glass electrode. The current-clamp protocols were applied by WinWCP V5.3.4 software (University of Strathclyde, UK). R-GECO1 fluorescence intensities were recorded using FIJI IMAGEJ-win64 software60.
Ca2+ signals were detected in N.âtabacum leaves utilizing the R-GECO1 reporter following Pst treatment using a perfusion system (780âμlâminâ1), which prevents motion and touch-induced imaging artefacts47,63,64,65. Pst inoculated from LBâagar plates containing 10âµgâmlâ1 rifampicin was cultured in LB medium with 10âµgâmlâ1 rifampicin for 1.5 days (28â°C, 200âr.p.m.) and subsequently subcultured in 500âml LB medium containing 10âµgâmlâ1 rifampicin for 16âh. Pst cells were washed twice using sterile deionized water and suspended in bath solution (1âmM KCl, 1âmM CaCl2, 10âmM MES and BTP, pHâ6.0), resulting in a final perfusion solution with an OD600nm of 0.5. Leaf disc samples from R-GECO1 transgenic N.âtabacum plants without abaxial epidermis were glued with the adaxial side down to the coverslip in custom-made chambers using Medical Adhesive B (Ulrich Swiss) and allowed to recover in bath solution overnight before Ca2+ imaging.
Membrane voltage recordings in mesophyll cells
Microelectrodes for mesophyll cell impalement were pulled from borosilicate glass capillaries (inner diameter 0.58âmm, outer diameter 1.0âmm, Hilgenberg) using a horizontal laser puller (P2000, Sutter). Microelectrodes filled with 300âmM KCl having an electrode resistance of 60â110âMΩ were connected by Ag/AgCl wires to the microelectrode amplifier (Axon geneclamp 500 or VF-102; BioLogic). Reference electrodes were filled with 300âmM KCl, and plugged with 2% agar in 300âmM KCl. The NA USB-6221 interface (National Instruments) was used to digitalize data. Cells were impaled by an electronic micromanipulator (NC-30, Kleindiek Nanotechnik) and current-clamp protocols were applied with the WinWCP V5.3.4 software (University of Strathclyde, UK).
Plant growth conditions and sample collection
All of the WT and transgenic N.âtabacum plants were grown under constant red light (650ânm, 30âμWâmmâ2, 26â°C) for 45âdays. For ACR1 2.0 and XXM 2.0 stimulation, 0âh, 1âh, 4âh and 24âh additional global green light (520ânm, 9âμWâmmâ2) were applied for the experimental groups. For osmotic stress treatment, 35% PEG was used to water Ret-eYFP #1 transgenic N.âtabacum plants. This PEG concentration was selected after initial experiments with different PEG concentrations that would cause wilting phenotypes in a similar time frame (after 4â5âh) to that for the green-light-treated Ret-ACR1 2.0 plants. For Pst treatment, the Pst was washed twice with sterile deionized water and suspended in 10âmM MgCl2 containing 0.04% Silwet L-77 with a final OD600nm of 0.5 and sprayed on the entire Ret-eYFP #1 transgenic N.âtabacum plants. Pst treatment was applied by spray inoculation to prevent wounding effects taking place that would unequivocally occur during infiltration by a syringe. The same amount (about 25âml for each plant) of 10âmM MgCl2 containing 0.04% Silwet L-77 was sprayed on Ret-eYFP #1 transgenic N.âtabacum as the negative control. At tâ=â0âh before the additional green light illumination, PEG, Pst or 10âmM MgCl2 was applied. For plants treated with 0âh, 1âh and 4âh green light illumination, the fifth leaves of N.âtabacum were collected at different time points (tâ=â0âh, 1âh, 4âh and 8âh) and frozen in liquid nitrogen quickly for metabolite measurement and transcriptomics analysis. For plants treated with 24âh global green light, PEG, Pst or 10âmM MgCl2, the fifth leaves from N.âtabacum at different time points (tâ=â0âmin, 3âmin, 10âmin, 0.5âh, 1âh, 2âh, 4âh, 8h and 24âh) were used for detection and quantification of ROS, electrolyte leakage estimations, chlorophyll fluorescence detection, metabolite measurement and transcriptomics analysis.
Detection of necrosis in leaf discs
A tissue puncher (Stiefel Disposable biopsy punch, diameter of 6âmm) was used to prepare leaf discs from the fifth leaf of 45-day-old N.âtabacum plants. Leaf discs were washed twice with deionized water and transferred into 24-well plates containing 0.4âml ultrapure H2O that contained 1âmM CaCl2, 10âmM CaCl2, 5âmM EGTA (pHâ7.0, KOH), 5âmM K4BAPTA or 5âmM K4BAPTA plus 10âmM NaCl as indicated in Extended Data Fig. 5c,d. Samples were placed in the dark for 1âh before exposing them to the light condition (growth chamber with constant red light (650ânm, 30âμWâmmâ2) plus constant green light (520ânm, 9âµWâmmâ2, 26â°C)). Images were captured after 24âh treatment.
Chlorophyll fluorescence measurements
N.âtabacum plants treated as described in the âPlant growth conditions and sample collectionâ section were used to quantify photosynthesis performance with a pulse-amplitude modulation fluorometer. The fifth leaf was fixed and monitored with a Maxi pulse-amplitude modulation fluorometer (AVT 033); chlorophyll fluorescence measurements were recorded with IMAGING WIN v.2.41a FW MULTI RGB (Walz). The dark-adapted N.âtabacum leaf was exposed to actinic light with intensity 7 (photosynthetically active radiation as 146âμmolâmâ2âsâ1). The maximal fluorescence yield of a dark-adapted sample (Fm) and dark-level fluorescence yield (Fo) were detected. The quantum yield of the dark-adapted leaf samples is a measure of the potential quantum yield of the samples, which was calculated according to the equation: Yieldâ=â(FmâââFo)/Fm.
Electrolyte leakage estimations
The membrane integrity of N.âtabacum leaf cells was estimated by electrolyte leakage of leaf samples. Ion conductivity was measured as described previously66. Seven leaf discs (5âmm diameter) were detached from the fifth leaves of 45-day-old plants and equilibrated together in 0.3âml of ultrapure H2O after washing twice with ultrapure H2O. Ion conductivity was quantified 20âmin after leaf disc equilibration in ultrapure H2O (EC1) using a LAQUAtwin EC-11 conductivity meter (Horiba). The samples were then heated at 99â°C for 1âh to measure the final electrical conductivity (EC2) when the samples reached room temperature again. The relative electrolyte leakage was calculated, as a percentage, by the formula: ELâ=âEC1/EC2âÃâ100.
Detection of ROS
Chemical detection of ROS in green light (520ânm, 9âμWâmmâ2)-treated N.âtabacum leaves was carried out by 3,3-diaminobenzidine (DAB, Sigma-Aldrich) staining as described previously67. The fifth leaves of N.âtabacum plants were stained with fresh DAB staining solution (10âmM Na2HPO4, 1âmgâmlâ1 DAB, 0.05% Tween 20, pHâ3.0) by application of negative pressure for 5âmin in dark. After 5âh incubation (shaking speed of 80â100âr.p.m.), the stained leaves were moved into fresh chlorophyll destaining solution (ethanol/acetic acid/glycerol, 3:1:1) and bathed in hot water (about 90â95â°C) for 15âmin. Finally, the stained leaves were put into cold fresh chlorophyll destaining solution for 30âmin. Images were taken with a plain white background under uniform lighting.
The chemiluminescent âsuperoxide probeâ luminol can be applied to indicate the ROS production68. Superoxide released from leaf tissues was detected by the luminescence of luminol with Skanlt software (Version 6.1) according to the method described previously69 with minor modifications. Leaf discs were prepared from the fifth leaves of 45-day-old N.âtabacum plants using a tissue puncher (Stiefel Disposable biopsy punch, diameter of 6âmm). Leaf discs were washed with deionized water twice and transferred into the 96-well assay plate (black plate, clear bottom with lid, Corning) and incubated in the dark overnight to recover. Water was replaced with 200âµl of luminolâperoxidase working solution (30âmgâlâ1 luminol (Sigma) and 20âmgâlâ1 horseradish peroxidase (Sigma)) in each well containing leaf discs. Samples were kept in the dark for 1âh before measurement. Luminescence was measured in a microplate reader (Luminoskan Ascent, Thermo Labsystems) and 5âmin global constant green light (9âµWâmmâ2) illumination was applied during the rest periods.
The method for in vivo measuring the production of H2O2 amperometrically from mesophyll cells in parallel with intracellular membrane potential recordings was described previously70. Measurements were carried out in standard bath solution (1âmM KCl, 1âmM CaCl2, 10âmM MES, and BTP, pHâ6.0) with a platinumâiridium electrode (MicroProbes) cut back to an active (uninsulated) area of about 1âmm length. ROS detection was carried out by Patch-Master software V2x90 (HEKA). The platinumâiridium disc was gently placed in close proximity to mesophyll cells and held at a constant voltage of 600âmV with an amperometry amplifier (VAâ10X, NPI Electronic). Oxidation of H2O2 at the active microelectrode surface resulted in a positive current signal, which was low-pass-filtered at 1âHz and recorded with Patch-Master software V2x90 (HEKA). The electrode was calibrated in freshly prepared bath solutions with defined H2O2 concentrations. Green light (520ânm, about 9âµWâmmâ2) illumination on the N.âtabacum leaves was carried out by green LEDs (WINGER, WEPGN3-S1).
All-trans retinal and carotenoid measurements
All-trans retinal and carotenoids were measured according to a protocol published elsewhere4. The fifth leaf of transgenic N.âtabacum plants grown for 45 days in red light was triturated in liquid nitrogen and 200âmg leaf material was extracted with 500âµl of chloroform. The extract was centrifuged for 5âmin at 18,400g and 50âµl of the organic phase was evaporated in a SpeedVac at 40â°C and dissolved in 50âµl of a 1:1 ethanol and chloroform mixture. A 5âµl volume of dissolved solution was analysed by ultrahigh-performance liquid chromatography (UPLC) combined with ultraviolet and tandem mass spectrometry detection using a Waters Acquity UPLC system coupled to a Waters Quattro Premier triple-quadrupole mass spectrometer equipped with an electrospray interface. Ten plants were used for retinal and 12 plants were used for carotenoid quantification.
Phytohormone measurement
All of the samples were prepared as described in the âPlant growth conditions and sample collectionâ section. Ground samples (150âmg) were lyophilized in a laboratory freeze dryer (CHRIST, Laboratory freeze dryer Alpha 1-2) and subsequently used for phytohormone extraction. The extraction and chromatographic separation was carried out as described previously71, using 5âng of dihydro-JA, JAânorvaline, [18O2]OPDA, [D4]SA and [D6]ABA as phytohormone internal standard. The extraction solution contained ethylacetate (p.a.) and formic acid (p.a.) (99:1 in volume) to which 5âng phytohormone internal standard was added. All samples were fixed on a TissueLyser with shaking for 3âmin at a speed of 23âHz. After that, samples were centrifuged and the supernatant was dried in a SpeedVac at 45â°C and finally dissolved in 40âμl liquid (acetonitrile (for high-performance liquid chromatography)/water (MilliQ), 1:1 (v/v)). Phytohormones were analysed by UPLCâelectrospray interfaceâtandem mass spectrometry using a Waters Acquity I-Class UPLC system coupled to an AB Sciex 6500+ QTRAP tandem mass spectrometer (AB Sciex), operated in negative ionization mode as described elsewhere72,73. Analyst (Version 1.6.3) software and MultiQuant (Version 3.0.2) software from Sciex were used for mass spectrometry detection of hormones and metabolites.
Proline quantification
The proline content of N.âtabacum leaves was measured according to the spectroscopic method of ref.â74. Samples were prepared as described in the section âPlant growth conditions and sample collectionâ. Ground samples (150âmg) were lyophilized in a laboratory freeze dryer (CHRIST, Laboratory freeze dryer Alpha 1-2). The dry samples were mixed in 40% ethanol and incubated at 4â°C overnight. The supernatant was collected after centrifugation at 13,500g for 5âmin. A 500âµl volume of the ethanol extraction or 100âµl standard solution was mixed with 1,000âµl reaction mix (1% ninhydrin (w/v) in 60% acetic acid (v/v) and 20% ethanol (v/v)). After incubation at 95â°C for 20âmin and subsequent centrifugation for 1âmin at 9,000g, the samples (supernatant) were subjected to absorption measurement at 520ânm with a spectrophotometer (Hitachi U-1500). Proline concentration was determined according to the standard curve, and concentrations were calculated on the basis of dry weight.
Transcriptomics analysis
Three replicates of leaf samples from two batches of N.âtabacum plants were collected for RNA sequencing. The experimental design for transcriptomics is shown in Extended Data Fig. 8a. Samples at 0âh, 1âh, 4âh and 8âh from plants growing in red light conditions were used as the biological controls to compare the expression levels of Ret-XXM 2.0 and Ret-ACR1 2.0 transgenic plants during or after the global green light illumination. In these experiments PEG-treated plants and Pst inoculation served as possible physiological controls to ACR1 2.0 and XXM 2.0 activation, and spraying leaves with buffer (MgCl2) served as an additional control to Pst-sprayed plants (Extended Data Fig. 8a). RNA extraction was carried out using ground samples (150âmg) with the Macherey-Nagel NucleoSpin RNA Plant Kit (https://www.takarabio.com/documents/User%20Manual/UM/UM_TotalRNAPlant_Rev_07.pdf). DNase1 (Thermo Fisher) was used to digest DNA. RNA sequencing was carried out by Novogene (UK) with an Illumina NovaSeq 6000 Sequencing System. Paired-end 150 bp was the read length. Data processing (fastp) and mapping to the N.âtabacum genome (kallisto)75 was carried out using Amalgkit (https://github.com/kfuku52/amalgkit). Functional annotations of N.âtabacum genes for subsequent bioinformatic analyses were retrieved from the Dicots PLAZA 5.0 repository76,77.
Normalization and DEG analysis were carried out employing the DIANE package using DESeq2 and default parameters77. DESeq2 uses a Wald test, in which the shrunken estimate of log fold change is divided by the standard error to produce a z-statistic. This z-statistic is then compared against a standard normal distribution78. A prefiltering step eliminated genes exhibiting rowMeans over all conditions ⤠5 counts, reducing the number of input genes from 69,500 to 42,196. Unless stated otherwise, |log2[fold change]|ââ¥â2 with a false discovery rate of 0.01 was taken as the cutoff for DEG identification. Only a small number of DEGs were identified when comparing Ret-ACR1 2.0 or Ret-XXM 2.0 transgenes with Ret-eYFP control plants grown under non-stimulating red light conditions (Supplementary Table 2, tab 1), demonstrating that ACR1 and XXM expression have virtually no impact on the transcript profiles of plants and plants growing in red light conditions are proper biological controls. Likewise, GO enrichment analysis on DEGs was carried out using the DIANE suite and corresponding enrichment plots were created using the srplot web interface (https://www.bioinformatics.com.cn/en). Venn diagrams were generated with the GOVenn script of the GOPlot package79 and subsequent GO analysis on Venn subsets was carried out using gprofiler280. g:Profiler functional enrichment analysis is conducted using the g:GOSt tool that carries out over-representation analysis via the hypergeometric test80. Finally, heat maps were generated with the pheatmap R package (version 1.0.12; https://CRAN.R-project.org/package=pheatmap). N.âtabacum genes were further annotated manually according to their A. thaliana orthologues81 and corresponding gene symbols from the Aramemnon database82.
Quantitative real-time PCR
Quantification of gene transcripts was carried out by real-time PCR as described elsewhere83. The samples were prepared as described in the section âPlant growth conditions and sample collectionâ. Ground samples (100âmg) were used for RNA extraction by the NucleoSpin RNA Plant Kit (Macherey-Nagel). cDNA was synthesized from 2.5âg of total RNA using oligo(dT) primer (Thermo Fisher Scientific) and M-MLV Reverse Transcriptase (Promega). All quantitative real-time PCR reactions were carried out with the Eppendorf Mastercycler ep realplex 2 system and Eppendorf Mastercycler ep realplex (Version 2.2) software, in a 20âµl reaction volume containing 2âµl diluted cDNA, 0.8âµM primer pairs and 10âµl ABsolute qPCR SYBR Green Capillary Mix (Thermo Scientific). Information on the genes and primers used is provided in Supplementary Table 7. Transcripts were normalized to that of 10,000 molecules of actin.
Surface potential recording on N.
tabacum leaves
The design for long-range electrical signal measurements in N.âtabacum leaves is shown in a diagram in Extended Data Fig. 10a. The surface potential recordings were carried out on 6â7-week-old N.âtabacum leaves according to a previously described protocol84 with minor modifications. A USB-6221 interface (National Instruments) was used to digitalize the electrical signals, which were recorded with WinWCP V5.3.4 software (University of Strathclyde, UK). The electrode silver wires (Ag/AgCl) connected to a microelectrode amplifier (Axon geneclamp 500) were wrapped around the petiole of the fifth leaves gently. Electrode gel (Auxynhairol) was used to cover the surface of the wrapped wires to aid connectivity between the electrodes and the petiole. The reference electrode consisting of an Ag/AgCl electrode was placed in a 200-ml pipette tip filled with electrode gel (Auxynhairol), which was inserted in the soil of the pots the N.âtabacum plants grew in. Nine hours after mounting the electrodes, the surface potential was recorded when applying a 600-ms green light (532ânm, 5.3âmWâmmâ2) pulse at the main vein. A popular Technology Enhanced Clad Silica multimode optical fibre (diameterâof 1,500âµm, 0.39 NA, Thorlabs) was placed directly on the top of main vein for light application. To prevent scattering of light and to guarantee local green light application, the optical fibre was covered by a non-transparent black plastic pipe up to the tip. The electrical signals were monitored at exactly 5âcm away from the illumination spot.
Significance analysis
Studentâs t-test or ANOVA was used to analyse significant differences between groups. Significance analysis among more than three groups was carried out with one-way ANOVA using IBM SPSS statistics (version 26.0). For the post hoc multiple comparisons, the homogeneity of variances was tested first. If the variances were homogeneous (Pâ>â0.05), the Tukey test was used for significance analysis. If the variances were not homogeneous (Pâ<â0.05), either the Dunnett T3 or Games-Howell test was chosen, depending on whether the sample sizes were equal or not. Different letters indicate significant differences among the samples (lowercase letters indicate P values at the 0.05 level and capital letters indicate P values at the 0.01 level). Significance analysis among two groups was carried out with a two-sided Studentâs t-test. *Pââ¤â0.05, **Pââ¤â0.01 and ***Pââ¤â0.001. The significance analysis is performed with a 95% confidence of interval. All of the P values are listed in Supplementary Table 8.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.