When Robert Hooke gazed through his microscope at a slice of cork and coined the term ‘cell’ in 1665, he was really looking at just the walls of the dead cells. The squishy contents typically found within would become objects of ongoing study. But for many plant scientists, the walls themselves faded into the background. They were considered passive containers for the exciting biology inside.
“For a long time, the cell wall was really thought to be dead,” says Alice Cheung, a plant molecular biologist and biochemist at the University of Massachusetts Amherst. It wasn’t until the late twentieth century, Cheung says, that scientists began to reveal the cell wall for the vibrant, ever-changing structure it is. Even then, its complex mix of sugar molecules linked into long, branching polysaccharides kept away all but the most intrepid biochemists.
Century-old genetics mystery of Mendel’s peas finally solved
But now, with the help of modern molecular methods to analyse the wall’s make-up and assembly, researchers are starting to uncover more. They’re finding that the cell wall is an active, even chatty participant in cellular growth, reproduction and responses to infection. It’s constantly receiving and sending signals about its shape and composition. By eavesdropping on those signals, and tweaking or adding to them, scientists are exploring innovative ways to improve agriculture with cell-wall science: protecting crops from disease and engineering new plants or sturdy hybrids.
“The plant cell wall is one of the most sophisticated systems in terms of communication,” says Li-Jia Qu, a plant biologist at Peking University in Beijing. His long-term goal is to use what’s been learnt about those messages to interbreed distantly related plants, creating exciting crops that could expand agriculture into new lands.
These walls can talk
The wall is the plant’s interface with its environment, including salt and other stressors or disease agents such as moulds, so it must notice damage and adapt accordingly.
The cell walls of a growing plant are constructed mainly from polysaccharides, including stiff cables of cellulose and jellified strands of pectin. The latter are highly complex molecules, branched in diverse ways and decorated with various extras such as methyl groups. “It’s like a giant bowl of many types of pasta, all mixed together,” says Charles Anderson, a plant cell biologist at Pennsylvania State University (Penn State) in State College.
And although the wall protects the contents within, some pathogens use enzymes to drill through it and infect the cells. This creates polysaccharide fragments that signal to the cell that something has breached the wall. When the cell senses these pieces, along with fragments of the infecting pathogen’s cell wall, it activates genes in the plant’s immune pathways. In response, the plant can produce an extra polysaccharide, called callose, which reinforces the cell wall. It also manufactures defensive molecules such as antimicrobial peptides and reactive oxygen species.
Such signals are already being co-opted by farmers. By spraying molecules derived from the cell walls of algae or fungi over their fields, they can prime the plants for pathogens that might arrive later. By doing this, they activate the immune response “and let the natural mechanism of the plant fight the infection”, says Antonio Molina, a plant biologist at the Technical University of Madrid. The method could help growers to avoid harsh fungicides, he says.
Molina has co-founded two companies that make use of this technique, making extracts from fungi or plants as crop protectives.
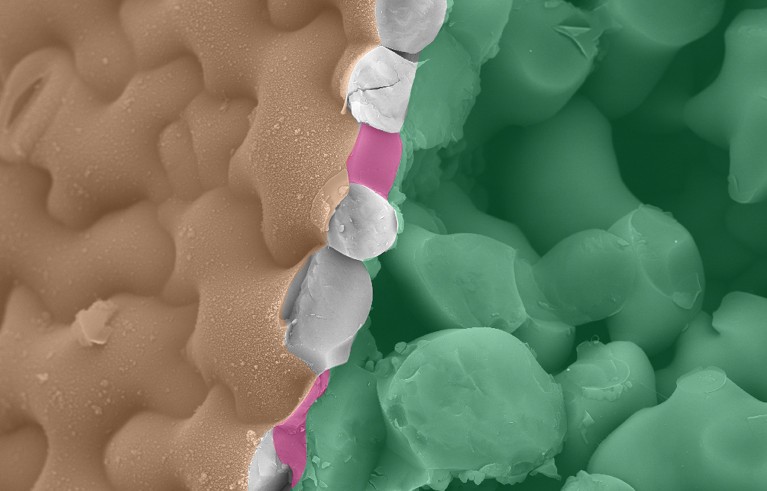
The puzzle-piece shape of these Arabidopsis thaliana pavement cells is created in part through signalling from the cell wall. Credit: Raymond Wightman/Sainsbury Laboratory, University of Cambridge and Alexis Peaucelle/INRAE
The current inoculants are fairly crude mixtures derived from plants or pathogens, says Cyril Zipfel, a plant immunologist at the University of Zurich, Switzerland. He’s working to understand the signalling that underlies the immune pathway, which should enable scientists to create more specific, or even synthetic, treatments.
There are trade-offs, however, Molina says. For one, the effects last for only three or four weeks. Reapplication for slow-growing crops could get costly, but Molina thinks that farmers might be able to focus the use of inoculants to times when risk of infection is high, such as after it rains, to prevent mildew.
Another challenge is that whenever plants devote resources to boosting their defences, this takes materials and energy away from growth, so farmers must apply the treatments judiciously.
Powered by pectin
Plant growth itself demonstrates how the idea of a cell wall as a static shell is insufficient. Yes, the cell does need the wall as a physical container; otherwise, it would burst from the enormous water pressure inside it. But for plant cells to grow, the cell walls must expand first, says Sebastian Wolf, a plant molecular biologist at the University of Tübingen in Germany.
This is where pectin comes in. Pectin is a complicated molecule constructed from at least a dozen sugars, connected by more than 20 types of linkage, says Wolf. “It’s actually so complex that we don’t know what it looks like,” he adds. Pectin is also dynamic, undergoing frequent modifications. Depending on those modifications, it can be rigid, supporting a sturdy plant, or softer when the plant needs to grow. That’s why pectin is often used to make marmalade: the initially soft pectin molecules cross-link and soak up water, taking on a stiffer, gelatinous consistency.
How CRISPR could yield the next blockbuster crop
In the growing plant, there’s one key modification that makes pectin soft or rigid: methyl groups attached to its component sugars. When the wall needs more materials for growth or reinforcement, the cell inside manufactures a methyl-decorated form that is thought to be fairly soluble, so it can be secreted into the surrounding wall. Once the pectin is incorporated into the wall, it starts to firm up. This happens when enzymes remove the methyls, uncovering negatively charged atoms in the sugar molecules underneath. Calcium ions in the wall bind to two sugars at a time and cross-link the pectin into a stiffer material that can absorb water.
As a graduate student at the University of Heidelberg, Germany, in the early 2000s, Wolf was interested in the effects of methyl groups on pectin, so he made a mutant of the cress Arabidopsis thaliana, a favourite of plant geneticists, that was unable to take off the methyl groups. He expected this to soften the cell walls, but the plants came out weirder than anticipated, with long, wavy roots1. They reminded him of mutants with defects in cellular signalling involving cellulose, leading him to wonder whether pectin, too, had a role not just in cell-wall structure, but also in cellular conversations.
Continuing this research at the French National Research Institute for Agriculture, Food and Environment in Versailles, Wolf discovered a cell-wall signal that contributes to plant growth controls1,2. The input that Wolf found starts when cell-surface receptors notice an overabundance of methyl-decorated pectin. What seems to happen in response is that they tell the cell to adjust its production pipelines, making more of the methyl–removing enzyme so that the wall can firm up the pectin.
Cell-wall signalling can even help growing cells to adopt fancy forms, including the puzzle-piece shapes seen in what are known as pavement cells — interlocking surface cells that give strength and structure to plant leaves (see ‘Cell walls fitting together’). When Anderson and his colleagues studied the signals sent by the cell wall as pavement cells developed in A. thaliana, they found evidence for another conversation initiated by methyl-free pectin and a receptor, called FERONIA, that senses this form of pectin3. But cellulose matters here, too. Both cell-wall components are needed to strengthen the indented portions of the puzzle piece, known as the ‘necks’. Without that fortification, the rest of the cell bulges into that space. If FERONIA isn’t present, the indentations don’t go as deep as they normally would.
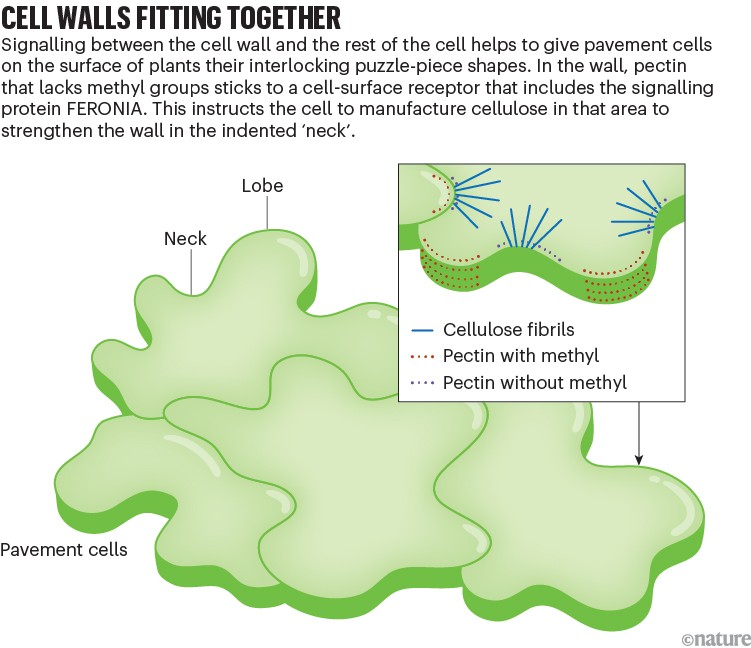
Here’s how they think the indentation process happens in a healthy leaf cell: methyl-free pectin in the cell wall is a sign that there’s enough pectin to support a neck. This pectin sticks to a receptor complex on the cell surface that includes FERONIA. In response, the cell starts manufacturing cellulose in the same place. Together, the cellulose and pectin strengthen the wall so that it can support the indentation.
Wolf is continuing to investigate the role of the cell wall in shaping cells, and reported in a preprint earlier this year that plant stem cells must control the methyl status of wall pectin to maintain their stemness and form new plant parts4. He thinks that there is potential to use these signalling pathways to influence the way in which plants are shaped. “You can fundamentally change how plants grow and how they look,” he speculates. For example, when plants make less cellulose, they grow into “stubby” forms. But, he cautions, researchers need to learn more about the underlying growth pathways first.
Hybrid potential
The FERONIA receptor in those puzzle-piece cells has turned out to be a key player in cell-wall signalling. FERONIA is found all over plants, and interacts with a range of partners to influence not just leaf-cell shape, but also a variety of other systems, from root growth to environmental-stress responses. Given that FERONIA binds to pectin to maintain the wall’s integrity, without it, the wall becomes weak and porous. Remarkably, mutants lacking FERONIA survive, although they’re pretty unhealthy, Qu says: they sprout wrinkled, curly leaves, and straggle along the ground because they lack the vasculature to hold them upright. They are “very small, tiny”, says Qu. They can make seeds, he adds, “but very few”.




