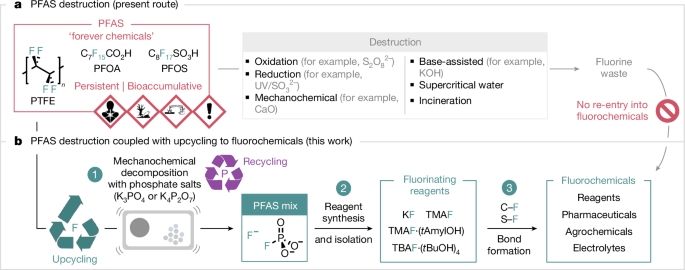
An observation made in the course of our study on the synthesis of fluorochemicals from fluorspar (CaF2) served as a starting point of investigation31. We noted that ball milling CaF2 with a phosphate salt (K2HPO4) in a stainless-steel jar with sealing rings made of PTFE (Teflon) instead of rubber gave higher yields of K3(HPO4)F and K2-xCay(PO3F)a(PO4)b, a new reagent for fluorination (Supplementary Information). This result suggests that fluoride leached from PTFE under these conditions, prompting the use of PTFE-free systems for CaF2 chemistry. This outcome was unexpected because C–F bond cleavage is mechanistically distinct from an ion exchange process. Methods for repurposing fluoroplastics, such as PTFE, are limited12,32,33,34 and involve harsh reaction conditions35; therefore, further investigation ensued (Fig. 2a). Ball milling PTFE with K3PO4 (1.25 equivalents (equiv.) per F) at 35 Hz for 3 h in a steel milling jar with a rubber sealing ring gave a solid material (PTFE mixKF) for which the water-soluble fraction was analysed by 19F nuclear magnetic resonance (NMR) spectroscopy (10% D2O in H2O). Signals at −120.6 and −73.2 ppm (1JPF = 867 Hz) ascribed to F− (84%) and PO3F2− (15%), respectively, indicated near-quantitative fluorine recovery. Alternative phosphate salts, including K2HPO4, KH2PO4 and K5P3O10, were less effective, but K4P2O7 stood out with the formation of a distinct mixture (PTFE mixPF) for which 19F NMR spectroscopy analysis (10% D2O in H2O) showed predominantly P–F bond formation (99% PO3F2−; δF = −73.7; 1JPF = 867 Hz) and only trace amounts of F− (less than 1%; δF = −120.1). Replacing the steel components (milling jar and balls) with zirconia gave close to identical results, and a control experiment confirmed that PTFE degradation is induced by the phosphate salt under the reaction conditions and is not linked to metal leaching from the steel components. For comparison, KOH (1.25 equiv. per F), which is known to decompose PFOA and PFOS by applying mechanical energy14,16, was found to be ineffective for releasing fluoride from PTFE (less than 10%) (Supplementary Information). These results prompted an in-depth investigation because potassium phosphate salts may offer a general and direct route to fluorinating reagents, such as KF, from PTFE and other PFASs, thereby contributing to a circular economy for the fluorochemical sector.
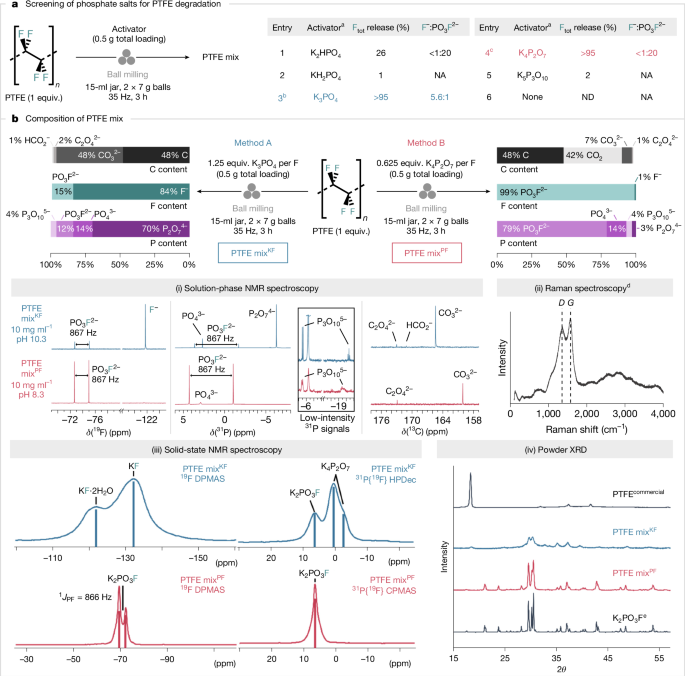
a, Screening of various phosphate salts as activators for PTFE. The total yield of released fluoride (both F− and PO3F2−) and their ratio were determined by quantitative 19F NMR spectroscopy (in 10% D2O in H2O using NaOTf as an internal standard). b, Identification of the C/F/P contents of PTFE mix by quantitative 13C/19F/31P NMR spectroscopy (in 10% D2O in H2O using KOAc or NaOTf as an internal standard) and Raman spectroscopy of water-insoluble black residue after aqueous extraction. Further analysis of PTFE mix by solid-state 19F/31P NMR spectroscopy and powder X-ray diffraction (XRD). aActivator stoichiometry was kept constant at 1.25 equiv. P per F. bReaction was also performed in a 15-ml zirconia jar with 2 × 6 g zirconia balls affording 98% Ftot release (F−:PO3F2− = 5.5:1). cReaction was also performed in a 15-ml zirconia jar with 2 × 6 g zirconia balls affording 99% Ftot release (F−:PO3F2− <1:20). dThe observed bands are denoted as D (disordered carbon) and G (graphitic carbon). ePrepared mechanochemically from KF and KPO3. NA, not applicable; ND, not detected.
Further analysis gave useful information on the composition of PTFE mixes (Fig. 2b and Supplementary Information). Quantitative 31P NMR spectroscopy (10% D2O in H2O) indicated that different ratios of PO3F2−, PO43−, P2O74− and P3O105− were present in the PTFE mixKF and PTFE mixPF. For the PTFE mixKF, P2O74− (δP = −6.3; 70% of total phosphorus (Ptot)) was the predominant phosphorus species, followed by PO43− (δP = 2.7; 14% Ptot), PO3F2− (δP = 1.0; 1JPF = 867 Hz; 12% Ptot) and P3O105− (δP = −5.5; −20.3; 2JPP = 20 Hz; 4% Ptot). In contrast, PO3F2− (δP = 1.5; 1JPF = 867 Hz; 79% Ptot) was the major species in the PTFE mixPF, followed by PO43− (δP = 2.9; 14% Ptot), P3O105− (δP = −5.5; −19.5; 2JPP = 20 Hz; 4% Ptot) and P2O74− (δP = −6.3; 3% Ptot). Quantitative 13C NMR spectroscopy gave insights into the fate of the carbon skeleton. The water-soluble fraction of the PTFE mixKF consisted mainly of CO32− (δC = 165.5; 48% Ctot), with trace amounts of C2O42− (δC = 173.0; 2% Ctot) and HCO2− (δC = 171.1; 1% Ctot). For PTFE mixPF, CO32− (δC = 160.2; 7% Ctot) was predominant, followed by C2O42− (δC = 173.0; 1% Ctot); HCO2− was not detected.
Taken together, these species accounted for only a fraction of the total carbon content of PTFE, prompting gas capture analysis. A significant quantity of CO2 (42% Ctot) was released in the case of K4P2O7-induced PTFE degradation, but no CO2 was detected with K3PO4, which is an advantage in the era of decarbonization (Supplementary Information). The water-insoluble black solid isolated from the PTFE mixKF and PTFE mixPF was analysed using Raman spectroscopy. Bands at 1,355 and 1,579 cm−1 were observed, consistent with the disordered and graphitic bands of carbon (48% Ctot for both samples)36. Solid-state 19F, 19F{31P} and 31P{19F} NMR spectroscopy showed that the PTFE mixKF contains KF, KF∙2H2O, K2PO3F and K4P2O7, whereas the PTFE mixPF is mainly composed of K2PO3F. The presence of K2PO3F in the PTFE mixKF and PTFE mixPF with no residual PTFE was confirmed by powder X-ray diffraction analysis.
Control experiments were performed to further understand this solvent-free mechanochemical process (Extended Data Fig. 1a). Both K3PO4 and K4P2O7 were subjected independently to ball milling in the absence of PTFE. No reactivity was observed for K3PO4, but minor quantities of K3PO4 (3%) and K5P3O10 (2%) were formed upon milling K4P2O7 (Supplementary Information). We also investigated the ability of the PTFE degradation products K2CO3, K2C2O4, K2PO3F and KHCO2 to decompose PTFE under otherwise similar ball milling conditions, demonstrating that K2CO3 was the most effective, yielding KF in 75%. These results indicate a complex mechanistic regime involving multiple oxyanion species. Further experiments demonstrated that ball milling K2PO3F with K3PO4 gave quantitative conversion to KF along with K4P2O7; when ball milled with K4P2O7, K2PO3F was mainly recovered, with 13% of KF being formed (Supplementary Information).
To gain insight into the ability of different oxyanions to decompose PTFE, we studied their global reactivity descriptors with ωB97xD/6-311++G(2d,2p) density functional theory (DFT) calculations (Extended Data Fig. 1b)37. Specifically, the global nucleophilicity index N38,39,40,41 was calculated and compared against the total yield of mineralized products KF and K2PO3F. A positive correlation suggests nucleophilic behaviour of the oxyanions in the milling process (Extended Data Fig. 1b(i)). Alternative theoretical formulations of the nucleophilicity indices yielded consistent results (Supplementary Information). A putative nucleophilic substitution reaction between various oxyanions and the model PFAS perfluorobutane was therefore examined next (Extended Data Fig. 1b(ii)). The reaction with K3PO4 favoured an SN2 transition structure at the internal carbon, with the lowest Gibbs free energy barrier (ΔG‡ = 31.8 kcal mol−1). K4P2O7 and K2CO3 gave the third and fourth lowest activation barriers (ΔΔG‡ = 9.3 and 10.4 kcal mol−1 versus K3PO4, respectively), demonstrating kinetic feasibility and the superiority of these three oxyanions for PFAS degradation. The ordering of computed activation barriers correlated with the experimental yields. For benchmarking, it is noteworthy that the weakest homolytic bond dissociation of perfluorobutane is 103 kcal mol−1 (ref. 42), which is comparable to the average C–C bond energy of 90 kcal mol−1 in PTFE43,44. Because we experimentally determined the composition of the PTFE mixKF (Fig. 2b), the standard enthalpy change (ΔHo) of an idealized process ([C2F4]n and K3PO4 leading to C, K2CO3, KF and K4P2O7; Supplementary Information) was determined as −143.5 kcal mol−1. This compared to a value of −107.9 kcal mol−1 for the decomposition of PTFE mixPF ([C2F4]n and K4P2O7 leading to C, CO2 and K2PO3F; Supplementary Information). The data are consistent with the favourable formation of KF (−2.95 eV per atom) and K2PO3F (−2.76 eV per atom) (Supplementary Information). Therefore, the phosphate-mediated decomposition of PTFE is enthalpically and entropically favourable.
The superiority of K3PO4 over KOH for the destruction of PTFE warrants further study of the solvation effect (that is, hydration). The putative SN2 reaction of perfluorobutane at an internal carbon with anhydrous KOH has the second lowest Gibbs free energy barrier (ΔG‡ = 34.3 kcal mol−1) of all oxyanions investigated. However, as expected, for mono- and dihydrate clusters of KOH, nucleophilic substitution is a kinetically more demanding process in comparison with anhydrous KOH (ΔΔG‡ = 8.9 and 16.3 kcal mol−1, respectively). Such solvation effects also impact K3PO4 but to a lesser extent (ΔΔG‡ = 5.5 kcal mol−1 (K3PO4∙H2O), 8.7 kcal mol−1 (K3PO4∙2H2O) and 12.8 kcal mol−1 (K3PO4∙3H2O)). Experimentally, inefficient PTFE decomposition was observed with KOH, irrespective of its water content (less than 10% fluoride release; Supplementary Information). By contrast, PTFE degradation was quantitative with anhydrous K3PO4, was poorly effective with K3PO4∙H2O (9% fluoride recovery) and did not occur with K3PO4∙3H2O. These data can be accounted for considering that the degradation of PTFE with KOH produces water, decreasing the hydroxide nucleophilicity through strong solvation as the reaction proceeds.
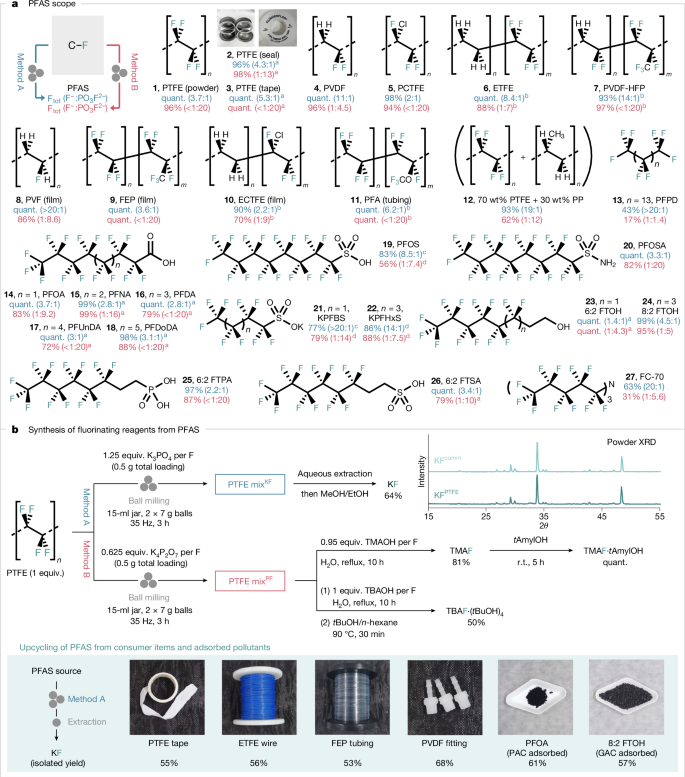
Having established the optimal conditions for the formation of fluoride and fluorophosphate from PTFE, we investigated the generality of this process with PFASs other than PTFE (Fig. 3a). The methodology was applied successfully to both polymeric (1–12) and non-polymeric PFASs (13–27); these include PTFE in various forms, including consumer Teflon seal and tape (1–3), PVDF (4), polychlorotrifluoroethylene (PCTFE) (5), ethylene tetrafluoroethylene (ETFE) (6), poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF–HFP) (7), polyvinyl fluoride (PVF) film (8), fluorinated ethylene propylene (FEP) film (9), ethylene-chlorotrifluoroethylene (ECTFE) film (10), perfluoroalkoxy alkane (PFA) tubing (11), a mixture of polypropylene (PP) and PTFE (12), perfluoropentadecane (PFPD) (13), PFOA (14), as well as long-chain derivatives (15–18), PFOS (19), perfluorooctanesulfonamide (PFOSA) (20), potassium perfluorobutanesulfonate (KPFBS) (21), potassium perfluorohexanesulfonate (KPFHxS) (22), 6:2 fluorotelomer alcohol (6:2 FTOH) (23), 8:2 fluorotelomer alcohol (8:2 FTOH) (24), 6:2 fluorotelomer phosphonic acid (6:2 FTPA) (25) and 6:2 fluorotelomer sulfonic acid (6:2 FTSA) (26). Noteworthy, the process also degraded FC-70 (27), which is a coolant liquid used in electronics.
a, Scope of PFAS destruction. The total yield of released fluoride (both F− and PO3F2−) and their ratio were determined by quantitative 19F NMR spectroscopy (in 10% D2O in H2O using NaOTf as an internal standard). Reactions were performed in triplicates, and average yields are reported. b, Synthesis of KF and tetraalkylammonium fluoride from PTFE and other PFAS sources under mechanochemical conditions. Yields of isolated products are reported. aReaction was performed once. bThe yield was calculated on the basis of the fluorine content of the copolymer as determined by elemental analysis. cThe amount of K3PO4 was increased to 2 equiv. per F. dReaction was carried out for 6 h. quant., quantitative; r.t., room temperature; TMAOH, tetramethylammonium hydroxide; TBAOH, tetrabutylammonium hydroxide.
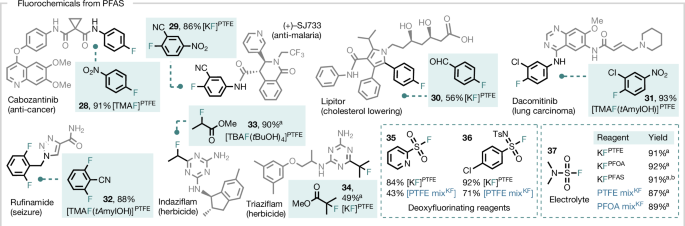
With a general method to destroy PFAS, we developed an efficient route to isolate the PTFE-derived potassium fluoride (KF) (KFPTFE) from the PTFE mixKF. The extractive purification of PTFE mixKF with H2O and MeOH/EtOH enabled the isolation of KFPTFE in 64% yield and 93% purity (calculated from PTFE; Supplementary Information). For comparison, the isolation of KF from PTFE ball milled with K2CO3 was more challenging and led to KF with lower yield (32%) and purity (55%). PTFE mixPF, resulting from the activation of PTFE with K4P2O7, served as the starting material for synthesizing tetramethylammonium fluoride (TMAF; 81%) upon treatment with tetramethylammonium hydroxide (TMAOH)45. TMAF was derivatized into its tert-amyl alcohol complex [TMAF·(tAmylOH)], a fluorinating reagent well documented for nucleophilic aromatic fluorination (SNAr)46. The bench-stable reagent tetrabutylammonium tetra(tert-butyl alcohol) fluoride [TBAF·(tBuOH)4] (50%) was prepared in a similar manner47. To demonstrate the applicability of this method, KF was successfully isolated from real-world consumer items, including PTFE tape, ETFE wire, FEP tubing and PVDF fittings. Furthermore, samples of PFAS adsorbed on powdered activated carbon (PAC) or granular activated carbon (GAC) were subjected to complete destruction, yielding KF for upcycling under identical conditions (Fig. 3b). All fluorinating reagents prepared from PTFE reacted as anticipated in the synthesis of various fluorochemical classes. KFPTFE performed comparably to commercial KF (KFcomm) in substitution chemistry, leading to 2,4-dinitrofluorobenzene (Supplementary Information). It was used to prepare 2-fluoro-5-nitrobenzonitrile (29) and methyl 2-fluoroisobutyrate (34), the precursors of (+)-SJ733 (anti-malaria) and triaziflam (herbicide), respectively. The deoxyfluorinating reagents PyFluor (35) and SulfoxFluor (36), as well as the electrolyte dimethylsulfamoyl fluoride (37) were also prepared in good yields from KFPTFE (Fig. 4). Notably, KF isolated from non-polymeric PFOA (KFPFOA) and other PFAS (KFPFAS), as well as crude mixtures (PTFE mixKF and PFOA mixKF) were efficient fluorinating reagents for S–F bond construction (35–37). The copper-mediated fluorination48 of potassium 4-formylphenyltrifluoroborate (prepared from KFPTFE) with KFPTFE afforded 4-fluorobenzaldehyde (30), a precursor of LIPITOR (cholesterol-lowering drug)49. Tetraalkylammonium fluoride reagents derived from PTFE mixPF were used to access 4-fluoronitrobenzene (28), 2-chloro-1-fluoro-4-nitrobenzene (31), 2,6-difluorobenzonitrile (32) and methyl 2-fluoropropanoate (33). These fluorochemicals are the precursors of cabozantinib (anti-cancer), dacomitinib (lung carcinoma), rufinamide (seizure) and indaziflam (herbicide) (Fig. 4).
Building block synthesis of high-value fluorochemicals using PFAS-derived fluorinating reagents (0.5-mmol scale unless otherwise stated). Yields of isolated products are reported. Detailed reaction conditions are stated in the Supplementary Information. aYield was determined by quantitative 19F NMR (in CDCl3 using 4-fluoroanisole as an internal standard). bKF was isolated from a mixture of decomposed PFASs, including PFOS, PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), PFOSA and 8:2 FTOH (Supplementary Information).
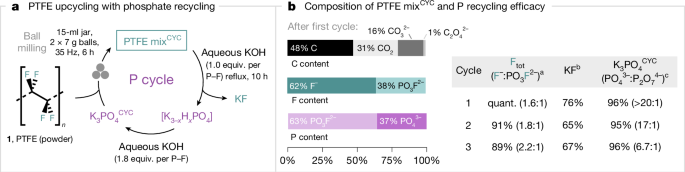
With a technology relying on K3PO4 for PFAS destruction, we considered its possible environmental impact on the phosphorus cycle, including wastewater contamination and anthropogenic eutrophication50. Therefore, our next goal was to establish that PTFE can be upcycled with a process enabling both the isolation of KF and the recovery of the phosphate salt for reuse (Fig. 5a). Because K4P2O7 was identified as the predominant by-product of PTFE mixKF and is itself an effective activator with formation of PTFE mixPF, the quantity of K3PO4 was adjusted for PTFE destruction coupled with KF recovery and K3PO4 recycling. Specifically, milling PTFE with K3PO4 (0.625 equiv. per F) at 35 Hz for 6 h in a steel milling jar gave a solid material (PTFE mixCYC) with quantitative fluorine release (F−:PO3F2− = 1.6:1), and no K4P2O7 was observed by 31P NMR. PTFE mixCYC was subjected to aqueous extraction to remove residual carbon black, followed by treatment with KOH (1 equiv. per P–F) at reflux for 10 h to ensure complete hydrolysis of K2PO3F. This process afforded KF isolated in 76% yield, along with K3PO4CYC recovered (96% of total P content) after further treatment with aqueous KOH (1.8 equiv. per P–F). K3PO4CYC is mainly composed of K3PO4 (85 wt%) along with traces of K4P2O7 (4 wt%). The performance of the recycled K3PO4CYC was maintained over two more cycles with effective KF and K3PO4 recovery (Fig. 5b).
a, Recovery of phosphate salts. b, Identification of C/F/P contents of PTFE mixCYC after first cycle and efficacy of recovered phosphate salts. Ball milling conditions: PTFE (1 equiv.; 86 mg) milled with K3PO4 (0.625 equiv. per F; 457 mg) or K3PO4CYC (457 mg) in a 15-ml stainless-steel milling jar, two chrome steel balls (2 × 7 g) at 35 Hz for 6 h. aThe total yield of released fluoride (F− and PO3F2−) as well as F−:PO3F2− ratio was determined by quantitative 19F NMR spectroscopy (in 10% D2O in H2O using NaOTf as an internal standard). bYields for isolated KF are reported. cThe total yield of recovered phosphate (PO43− and P2O74−), as well as PO43−😛2O74− ratio was determined by quantitative 31P NMR spectroscopy (in 10% D2O in H2O using PO(OEt)3 as an internal standard); recovered phosphate salts contain up to 8% carbonate.