The replacement of lost DA neurons with hfVM in patients with PD began in the 1980s22,23. Although PS cells are expected to be an alternative donor cell source to hfVM, the safety and efficacy of iPS-cell-derived DA progenitors remains unclear. This first clinical trial using iPS cells confirmed that iPS-cell-derived DA progenitors can survive without forming tumours and produce DA in the putamen of patients with PD. Moreover, there were no serious adverse events or GIDs. Four out of six patients showed improvement in MDS-UPDRS part III OFF at 24 months after transplantation, suggesting that grafted cells functioned as DA neurons.
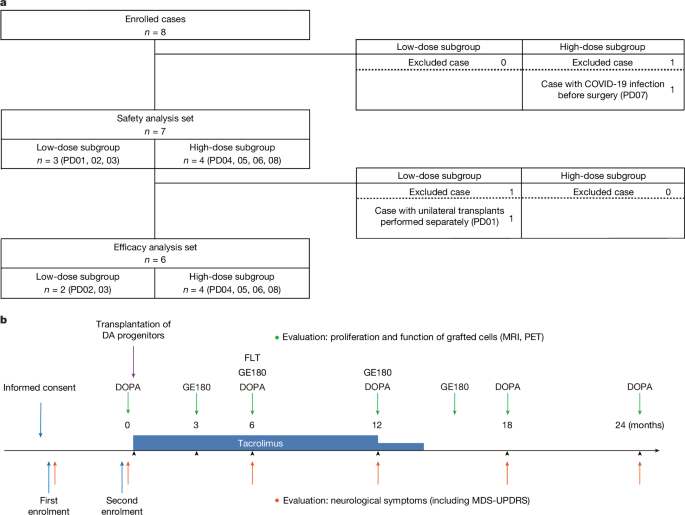
No serious adverse events were reported for all seven patients subjected to safety assessment. The graft size measured with MRI T2-weighted images gradually increased over the 24 months. However, no tumorigenic overgrowth was identified, as evidenced by the absence of 18F-FLT uptake, a marker of cellular proliferation, which was indirectly supported by results from the transplantation experiment using the same donor cells into PD model rats. Histological analyses at 24 or 32 weeks showed no tumour-like overgrowth, with less than 1.0% of cells being Ki-67 positive. Rather than proliferation, a previous animal study reported that the apparent increase in graft volume was due to the spread of the grafted cells9. While such effects may also apply to our trial, further confirmation through long-term follow-up and post-mortem histological examinations is necessary. The spectrum of adverse events was similar to those encountered with chronic DA replacement medication, tacrolimus administration and brain surgery. Neck stiffness and painful dystonia in the right upper limb were noted in PD01 during the drug-on state, a phenomenon possibly related to the grafts. Tacrolimus- and surgery-related adverse events were manageable and reversible.
One critical concern about hfVM transplantation was GIDs4,5,24. In this trial, six out of seven patients showed a mild worsening of dyskinesia, resulting in an average increase in the UDysRS total score of 12.3 points (116.4%) from the baseline at 24 months, probably because anti-parkinsonian medication doses were maintained throughout the trial, except when therapeutic adjustments were necessary. The protocol was designed to minimize the impact of medication changes. Consistently, patients recorded dyskinesia in both upper and lower extremities during the on-time period. This clinical presentation is typical for drug-induced dyskinesia and not for GIDs, which occur during the off-time and are often observed predominantly in the lower extremities4,5,24. This suggests that grafted cells replicate the effects of levodopa, including the tendency to induce dyskinesias in susceptible patients. Alternatively, a focal rather than diffuse distribution of DA stimulation from the graft may exacerbate dyskinesia25. In previous reports, GIDs were attributed to serotonergic neurons contained in hfVM tissue26,27,28. In our DA progenitor preparation, we purified CORIN+ medial plate cells and eliminated lateral plate cells, which include serotonergic neurons. As a result, we detected no 5-HT+ cells in donor-cell-derived grafts in rat PD models. This purification process may have contributed to the absence of GIDs in our trial.
The appropriate regimen of immunosuppression in allogeneic neural transplantation remains controversial. Previous clinical trials used a different combination of immunosuppressant drugs, including ciclosporin, azathioprine and prednisolone4,5,6,29. Our previous non-human-primate studies demonstrated no acute immune response after allogeneic transplantation of monkey iPS-cell-derived DA progenitors without immunosuppression30. Furthermore, tacrolimus alone effectively suppressed immune responses during both allotransplantation (monkey to monkey)31 and xenotransplantation (human to monkey)12. On the basis of these findings, we used tacrolimus as the sole immunosuppressant in our clinical trial. Histological analyses from previous fetal cell transplantation studies have shown that grafted DA neurons can survive for 9 to 16 years, even when immunosuppression is discontinued 6 to 18 months after transplantation32,33. Moreover, our positron-emission tomography (PET) study at 3, 6 and 12 months showed no 18F-GE180 uptake, suggesting the absence of severe inflammation. As a result, we discontinued tacrolimus treatment at 15 months. After terminating immunosuppression, no inflammation due to immune response was observed in the putamen and surrounding areas, as evident by the absence of hyperintensity regions on T2-weighted and FLAIR MRI or increased 18F-GE180 uptake. Moreover, there was no clinical difference between HLA-matched and non-matched patients. However, further confirmation through long-term follow-up and post-mortem histological examinations is necessary for definitive conclusions.
Our results demonstrated a beneficial effect on MDS-UPDRS part III during both on- and off-time periods. Specifically, PD02, PD04 and PD08 showed improvements of 32.4%, 45.1% and 50.0% during off-time at 24 months, respectively, while PD03 showed a 9.1% improvement. Considering the increase in 18F-DOPA uptake, these results suggest that grafted cells function as DA neurons, therefore functionally replacing lost DA neurons. As this is an open-label trial without a control group, it is important to consider the potential influence of the placebo effect and observer bias. A systematic analysis of placebo responses (placebo effect plus observer bias) in nine double-blind, randomized trials of regenerative therapies for PD reported an average improvement of 4.3 points in MDS-UPDRS part III OFF scores, with a 95% confidence interval of 3.1 to 5.6, with a mean observation time of 11.3 months34. Moreover, a PET study conducted in four out of nine trials showed no significant increase in 18F-DOPA uptake in sham-operated groups. Furthermore, the placebo effect in patients with PD is thought to be mediated by the release, rather than synthesis, of endogenous DA in the striatum35. On the basis of these findings, at least three patients (PD02, PD04, PD08) in this trial exhibited motor symptom improvements exceeding what could be attributed to placebo responses, potentially due to DA synthesized by the graft. This interpretation should be further validated through post-mortem histological examinations in the future.
Among the three patients (PD02, PD04 and PD08) who exhibited a beneficial effect on MDS-UPDRS part III, only PD02 showed improvement in both the off-time period and PDQ-39 scores. These evaluations are subjective and reflect the patient’s perceptions. It is possible that patients had very high expectations for this new treatment, and the results did not meet such high expectations despite objective improvements. In the remaining two patients (PD05 and PD06), motor deficits stabilized at a similar level of decline to those receiving conventional medication36,37. These two patients exhibited a higher degree of deterioration in motor symptoms compared with the other patients during the on-time, suggesting that faster neurodegeneration, especially in the non-dopaminergic systems, diminished the beneficial effects produced by the graft during the trial period. Notably, PD05 was 69 years old at the baseline, and as previous studies on fetal transplants suggest4,5, younger patients with less severe symptoms appear to be more suitable candidates for this treatment. Considering these results, refining patient eligibility criteria may enhance the efficacy of this treatment.
In some cases, especially PD03 and PD06, discrepancies were noted between the MDS-UPDRS part III scores and the Hoehn–Yahr stage. The Hoehn–Yahr stage emphasizes postural instability and mobility issues, whereas the MDS-UPDRS part III offers a more comprehensive evaluation of major motor symptoms in PD. Consequently, improved postural stability and mobility may account for the greater improvement in the Hoehn–Yahr stage compared with changes observed in MDS-UPDRS part III scores of this study.
The substantial increase in putaminal 18F-DOPA uptake in the high-dose group compared with the low-dose group suggests dose-dependent characteristics. Plots of pre-transplant and post-transplant Ki values and MDS-UPDRS part III OFF scores revealed a mild overall trend but no clear correlation at the individual level (Extended Data Fig. 6i,j). This lack of correlation may be due to the complexity of cell replacement therapy: 18F-DOPA uptake does not necessarily reflect the activation of postsynaptic neurons, and motor symptoms are influenced by both dopaminergic and non-dopaminergic neural circuits. The functional impact of grafted cells probably requires more than just DA delivery. Successful integration of the graft into the host brain is crucial for achieving meaningful clinical recovery in PD38. Another possibility is that the absolute Ki value is more important than its level of increase and that the number of surviving DA neurons is still insufficient. Previous studies have reported that Ki values of healthy individuals range from 0.010 to 0.017 (with our experience indicating a range of 0.010 to 0.015). While Parkinsonian symptoms typically emerge when Ki values decrease by 41% to 58% from the normal range39,40,41, even the highest Ki value observed at 24 months fell within the range associated with the onset of initial symptoms. Furthermore, 3 out of 12 putamen samples (PD02R, PD02L, PD06L) did not show an increase in 18F-DOPA uptake (Extended Data Fig. 6a,b). This may be due to the technical limitation of measuring uptake in the whole putamen. However, PET images revealed distinct uptake at the injection sites (Extended Data Fig. 6c–h), which may have contributed to symptom improvement. Importantly, there was no difference in adverse events between low- and high-dose groups, and neither graft overgrowth nor GIDs were observed, even in the high-dose group. Considering these results, implanting more cells across a broader area may be necessary to achieve more substantial therapeutic effects. The favourable safety profile observed in this trial provides an opportunity to explore whether a higher dose across a wider region can offer greater clinical efficacy.
Previous open-label trials using human hfVM demonstrated that cells engrafted into the host striatum synthesized DA and improved motor symptoms. In favourable cases, symptom improvement persisted for over 10 years without severe adverse events33,42. While two double-blind, placebo-controlled trials did not find significant differences between the graft and control groups, they did show significant motor symptom improvement in specific subgroups. These findings suggest that cell replacement therapy may be beneficial if appropriate patients are selected. One beneficial subgroup may be patients 60 years old or younger, while another included those with less severe stages (below 50 points as evaluated by the original UPDRS part III OFF score43). Although our results did not fully align with age-related findings, it is notable that the worst case (PD05) was the oldest patient, and the best case (PD08) was the youngest. In terms of MDS-UPDRS part III OFF scores, patients PD02 and PD08, both with scores under 50, demonstrated symptom improvement.
As discussed above, this trial has certain limitations. First, for definitive conclusions regarding the survival of mature DA neurons, inflammation around the graft, and tumorigenicity, post-mortem histological analyses are required. Second, eligibility criteria for optimal patients with PD receiving cell replacement therapy have yet to be defined. Third, graft-derived reinnervation may cover only some DA-depleted striatal regions2. These latter factors may partially explain the variable clinical responses observed in this trial. Fourth, this was an open-label trial, susceptible to influence from the placebo effect and physician bias. Future studies should consider a double-blind, placebo-controlled design to minimize these biases. Lastly, the findings of this single-centre, small-sample trial should be confirmed in multi-centre, large-sample trials with appropriate controls.
In conclusion, while the safety and efficacy of iPS-cell-derived cell products continue to be investigated, this trial demonstrated the safety profile of iPS-cell-derived DA progenitors. After bilateral putaminal transplantation, the average motor severity was decreased and the mean 18F-DOPA uptake was increased at the 24-month follow-up. Despite the abovementioned limitations, these findings suggest that allogeneic transplantation of iPS-cell-derived DA progenitors is a safe and effective regenerative therapy for patients with PD. Future strategies may combine cell transplantation with gene therapy, medication and rehabilitation to enhance efficacy44. Moreover, as demonstrated in a single case study8, autologous transplantation using iPS cells may also be a promising option.