Statistical analysis
Unless otherwise stated, we used the following system to indicate significance levels in the figure panels: *P < 0.05; **P < 0.01; ***P < 0.001. Statistical tests used are indicated in the main text or figure caption, with specific tests for chromosome biases and breakpoint as well as SCE locations detailed in the Supplementary Methods.
Cell culture and cell line development
MCF10A (CRL-10317, American Type Culture Collection) and RPE-1 (CRL-4000, American Type Culture Collection) cell line and their TP53−/− derivatives were cultured at 37 °C with 5% CO2 atmosphere and 100% humidity, in DMEM/F12 medium (1:1) without phenol red (Gibco), supplemented as follows: RPE-1 medium was further supplemented with 10% FCS, 2 mM l-glutamine (Gibco) and antibiotics; MCF10A medium with 5% horse serum (Thermo Fisher Scientific), 2 mM l-glutamine (Gibco), 20 ng ml−1 human EGF (Biotrend), 0.5 mg ml−1 hydrocortisone (Sigma-Aldrich), 100 ng ml−1 cholera toxin (Sigma-Aldrich), 10 μg ml−1 recombinant human insulin (Sigma-Aldrich) and antibiotics. BJ-5ta (CRL-4001, American Type Culture Collection) were cultured at 37 °C with 5% CO2 atmosphere and 100% humidity in a 4:1 ratio of DMEM (Gibco) and Medium 199 (Gibco) without phenol red, supplemented with 10% FCS, 2 mM l-glutamine (Gibco) and antibiotics. IMR-90 (CCL-186, American Type Culture Collection) were cultured at 37 °C with 5% CO2 atmosphere and 100% humidity in Minimum Essential Medium containing Earle’s salts and without phenol red (Gibco), supplemented with 10% FCS, 2 mM l-glutamine (Gibco), 1 mM sodium pyruvate (Gibco), 1× NEAA (Gibco) and antibiotics; cells were discarded after 15 population doublings. MCF10A TP53−/− cells were kindly provided by C. Scholl (Laboratory of Applied Functional Genomics, DKFZ), whereas RPE-1 TP53−/− variants were generated in a previous study from our laboratory63. All cell lines tested negative for mycoplasma contamination.
For experiments using H2B-Dendra2 as photolabelling strategy, a plasmid carrying H2B-Dendra2 (ref. 64) (Addgene, plasmid no. 75283) was introduced by transfection: 20,000 cells were seeded in a glass-bottom slide (Nunc LabTek eight-well) and transfected with 20 µl of transfection mixture at 4:1 ratio of Fugene HD (Promega) to DNA in Opti-MEM (Thermo Fisher Scientific). Transfection success was assessed 48 h later by fluorescence microscopy, cells were transferred into two 10-cm dishes and G418 antibiotic was added at 200 µg ml−1 (MCF10A) or 400 µg ml−1 (RPE-1) for selection. Two weeks later, well separated, fluorescent colonies were visible and were isolated by pipetting, transferred to 24-well plates and grown into stable cell lines. Stable-transfectants for RPE-1 wild-type and RPE-1 TP53−/− were instead collected in pool and isolated by single-cell sorting using a BD FACSAria at 1.0 flow rate, with a 130 µm nozzle, dispensed in a flat-bottom, 96-well plate (Thermo Fisher, Nunc plates) with normal growth medium. In experiments designed to induce micronucleus formation biochemically, MCF10A and RPE-1 cells were treated with 0.5 µM of reversine (Sigma), a potent MPS1 inhibitor65, 1 day after seeding. After 24 h of treatment, cells were washed gently four times with 1× PBS before being released into fresh medium.
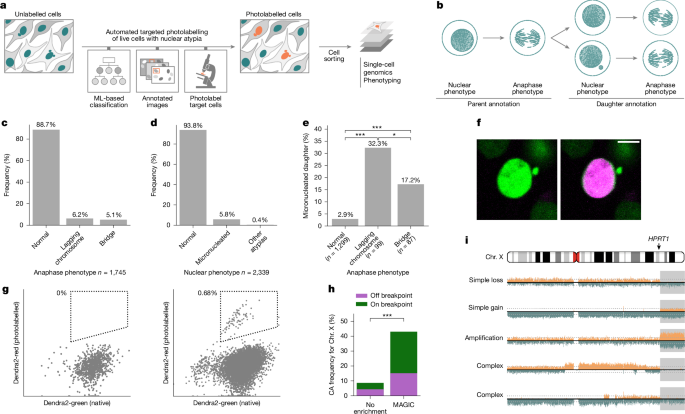
MAGIC: autonomous platform for de novo CA formation studies
MAGIC leverages machine learning and automated microscopy to perform targeted photolabelling of cells of interest, for subsequent fluorescence-activated cell sorting and downstream analysis, building on approaches coupling the imaging of visual phenotypes with precise optical tagging66,67,68. An MAGIC experiment with this adaptive feedback microscopy (Smart Microscopy) system comprises three phases: (1) the preparation phase of MAGIC, where cells are seeded and other treatments, such as targeted DSB induction or staining by DACT-1, can take place; (2) the photolabelling phase of MAGIC, where targeted illumination66,67,69 takes place using automated microscopy and (3) the cell collection phase of MAGIC, when cells are collected and isolated by FACS. These steps are outlined below, accompanied by further details presented in the Supplementary Methods.
Preparation
During this phase cells are prepared to undergo the targeted photolabelling procedure. Further treatments, such as targeted DSB induction, staining with live-cell dyes and adding BrdU for Strand-seq, can take place. To enable photolabelling, we engineered MCF10A and RPE-1 cell line models to constitutively express H2B-Dendra2—a monomeric fluorescent protein that undergoes irreversible photoconversion with 405 nm light, which also enables the visualization of nuclear atypia without affecting mitotic fidelity70. As an alternative, for RPE-1 wild-type cells, as well as BJ-5ta and IMR-90, we also used DACT-1—a photo-activatable cell tracking dye—that converts to a bright red-fluorescent state upon 405 nm light exposure (further details are available in the Supplementary Methods). Neither H2B-Dendra2 nor DACT-1 significantly altered micronucleus frequency in MCF10A cells (Supplementary Fig. 1f), indicating that these labelling approaches, by themselves, do not induce chromosomal instability under the conditions used.
Cells were seeded in up to four wells of a µ-slide eight-well dish (Ibidi). Seeding density was adjusted to have about 40,000 cells 1 day before experiment start. In the case of Strand-seq downstream analysis, BrdU (40 µM final concentration) was added to the cells before the start of photolabelling (a concentration previously reported not to cause genomic instability71). One control slide without BrdU was also prepared to adjust gating strategies during single-cell sorting. In the case of targeted DSB induction experiments, ribonucleoprotein (RNP) complexes were delivered by electroporation 48 h before the start of the experiment and up to two different sgRNAs were examined during a single experiment.
Photolabelling
Living cells were then transferred to an LSM 900 microscope (Zeiss) with confocal and widefield imaging capabilities, and an environmental chamber with temperature and CO2 control. MAGIC relies on full microscope automation and computer vision for laser-assisted, phenotype-driven targeted illumination of single cells at scale. The system includes three software components: a microscope control script, an image analysis manager on the basis of AutoMicTools and a Python package, magic_tools, which we designed for advanced image processing.
The microscope control script automates autofocusing, micronuclei identification and photoconversion of target nuclei across several positions. Autofocus is achieved by detecting the glass-bottom dish reflection using a 639 nm laser and AutoMicTools analysis. For micronuclei identification, a Z stack image centred on the focused slice is analysed on an image analysis server driven by magic_tools. Photoconversion involves using micronuclei coordinates to define ROIs of the corresponding parental nuclei, which are then photolabelled selectively with a 405 nm laser. Pre- and post-experiment images are acquired before the microscope moves to the next position.
We ran the photolabelling experiment overnight and up to 24 h, to achieve a yield of 700 to 2,000 photolabelled cells, depending on the experimental conditions. A detailed description of the automation software and the image analysis pipeline can be found in Supplementary Methods.
Cell collection
Following photolabelling, cells were collected and target cells were isolated by single-cell sorting. In case of Strand-seq experiments, at the end of the photolabelling phase, cells were stained for 1 h with Hoechst 33342 at 5 µg ml−1. Cells were collected with 0.25% trypsin (Gibco) and resuspended in buffer (8% FBS in 1× PBS, supplemented with Hoechst 33342 5 μg ml−1 and BrdU 40 µM). Single cells were sorted using a BD FACSAria in purity mode with a 100-µm or 130-µm nozzle and dispensed into lysis buffer or fresh medium in a flat-bottom 96-well plate (Thermo Fisher, Nunc plates). We used the following gating strategy: we selected first the general population in forward and side scatter and we excluded doublets. Then, cells were sub-gated for photolabelled cells as shown in Fig. 1g for H2B-Dendra2 or Supplementary Fig. 2h for DACT-1. When using Strand-seq, the singlet population was further filtered to select cells with a quenched Hoechst signal that had thus incorporated BrdU72. Cells collected from control slides were used to optimally adjust gates to exclude false positives.
Long-term live-cell imaging
The live-imaging experiment for nuclear and mitotic phenotype16,73 scoring was carried out over the course of 72 h. MCF10A cells stably expressing H2B-Dendra2 were seeded at a 15–25% confluence on µ-slide eight-well dishes (catalogue no. 80806; Ibidi), and images were acquired every 10 min with a Plan-Apochromat ×20/0.8 M27 air objective using the LSM 900 confocal microscope (Zeiss). Manual annotation was performed with the assistance of a customized tool written in Python. Mitotic phenotype and nuclear morphology for parental cells and the first generation of daughter cells were annotated as described in Fig. 1b.
Optimization of photolabelling parameters
MCF10A cells stably expressing H2B-Dendra2 were seeded on μ-slides (Ibidi) and imaged on an LSM 900 confocal microscope (Zeiss). To determine Dendra2 photoconversion dynamics, we performed five bleaching rounds, each with ten laser-scanning iterations with a ×20 objective and 405-nm laser, at scanning speed 8 and power at 0.5% in the low-intensity power range. Images in green and red channels were acquired at the beginning and end of each round. The fluorescence intensity of ten photoconverted nuclei and five non-photoconverted control nuclei per field of view was quantified on manually defined ROIs with ImageJ. Data were then processed and analysed with custom Python scripts. To assess phototoxicity from targeted illumination, MCF10A and RPE-1 cells seeded on μ-slides (Ibidi) were photoconverted with settings used in the MAGIC pipeline and followed by confocal microscopy. Images for native and photoconverted Dendra2 fluorescence channels were acquired with a ×20 objective over the course of 24 h. Cells were tracked manually and their fate annotated. No cell death was detected for the photoconverted cells within the timeframe analysed.
Single-cell genomic sequencing with Strand-seq
Unlike other single-cell genomic techniques, Strand-seq uniquely preserves haplotype identity across an entire homologue27,29, which enables sensitive detection of simple and complex CA classes at intermediate sequence coverage29,74. We performed cell sorting as in the original procedure27 with important adjustments to accept whole cells as input, to avoid loss of cytoplasmic DNA material and micronuclei during nuclei isolation. Cells were incubated with Hoechst 33342 (5 μg ml−1) for 60 min, as it is cell membrane-permeable. Cells were then collected with 0.25% trypsin (Gibco) and resuspended in buffer (8% FBS in 1× PBS, supplemented with Hoechst 33342 5 μg ml−1 and BrdU 40 µM). Single cells were sorted using a BD FACSAria in purity mode with a 100 or 130 µm nozzle, and dispensed into a flat-bottom 96-well plate (Thermo Fisher Scientific, Nunc plates) containing freeze buffer supplemented with 0.2% NP-40 (Thermo Fisher Scientific) to ensure membrane lysis and DNA accessibility in subsequent protocol steps. Strand-seq libraries were prepared at large-scale using a liquid handling robotic platform as described previously29. Libraries were sequenced on a NextSeq5000 (MID-mode, 75 bp paired-end) followed by demultiplexing. Reads were aligned to GRCh38 reference assembly with BWA-MEM v.0.7.17, yielding a median of ~285,000 mapped unique fragments per cell, and further processed as described below.
Single-cell de novo CA discovery and classification
We discovered a wide variety of de novo CA classes leading to chromosomal or segmental copy-number imbalances by integrating read coverage and Watson/Crick template ratios29, enabling high-resolution CA calling in Strand-seq data. Extending the functionality of the previously released MosaiCatcher tool29, we designed strandtools, which is tailored for the specific task of handling de novo CA discovery in single cells under diverse ploidy backgrounds (Supplementary Methods). To achieve high confidence CA classification, we integrated read depth, strand orientation and haplotype information in each cell29, to characterize segmental alterations and assign them to one of the following CA classes: chromosome loss, chromosome gain, interstitial loss, interstitial gain, terminal loss, terminal gain, terminal multi-step, complex CA and chromothripsis (a complex CA subclass). Chromosome gains and losses affect a whole chromosome, from p-ter telomere to q-ter telomere. Interstitial gains and losses are isolated CAs between two breakpoints, within one chromosome arm. As terminal alterations, we refer to all CAs that involve a portion of a chromosome, from a breakpoint anywhere along a chromosome arm to the telomere of that same arm. Therefore, terminal gains and losses are simple CAs, with one isolated, altered segment spanning from a breakpoint to the telomere of one chromosome arm. Terminal gains are annotated as inverted duplications if the gained segment is in opposite strand orientation compared with that of the original homologue with the same haplotype29. Terminal multi-step CAs are a sequential combination of gains and losses that are affecting the terminal portion of a chromosome arm. The terminal multi-step class also includes all cases of localized oscillations arising alongside terminal gains and losses.
Complex CAs are defined as events that include more than two breakpoints, can affect either one or both arms of the same homologue and can be composed of non-adjacent, altered segments. As such, complex CAs cannot be resolved as terminal multi-step. Chromothripsis events extending over large chromosomal regions, such as a chromosome arm, are included under the complex CA class. These events show characteristic copy-number oscillation between typically two copy-number states, affecting one single haplotype and with oscillating segments allowed in either strand orientation29,30. With regard to experiments on targeted DSB induction along chromosome arms, we likewise considered all copy-number imbalanced CA classes. In addition, we specified whole-arm alterations in the case of isolated gains and losses affecting more than 90% of a chromosome arm, and amplifications in case of isolated gains with a copy-number increment of two or more compared to the baseline. All single-cell CA annotations are available in Supplementary Tables 7, 8 and 9.
Targeted induction of DSBs
CRISPR components, designed as described in the Supplementary Methods, were delivered in the form of RNP complex using a Neon Electric Transfection System (10 µl kit; catalogue no.: MPK1096; Thermo Fisher). First, the RNP complex was formed by incubating 0.3 µl of Alt-R S.p. Cas9 Nuclease (catalogue no.: 1081059; IDT) with 0.2 µl Resuspension Buffer R (Neon 10 µl kit) and 1 µl of designed sgRNA for 20 min. Cells (500,000 per reaction) were prepared for electroporation as described in the manufacturer’s manual. Concentration of Cas9 nuclease in the final RNP/cell suspension was 1.5 µM, and that of sgRNA was 3.6 µM. Electroporation parameters of 1,400 V, 20 ms and two pulses were used for both RPE-1 and MCF10A cells. Transfected cells were diluted in antibiotic-free cell culture medium and different amounts (between 36,000 and 72,000) were seeded into four central wells of µ-slides containing 300 µl of antibiotic-free medium. The medium was replaced with fresh medium containing BrdU (40 µM) at 48 h post-transfection to allow cells to recover, and the slide was transferred immediately into the confocal microscope for imaging. For determining how DSB location may determine CA processes, we selected chromosome 2q due to its low average repeat content facilitating gRNA design, and 7q due to the enrichment for clonally propagated CAs we observe for this arm.
Clone generation from single cells
Cells were subjected to automated photolabelling, collected with 0.25% trypsin (Gibco) and resuspended in buffer (8% FBS in 1× PBS). Single cells were sorted using a BD FACSAria at 1.0 flow rate, with a 130-µm nozzle to minimize cell damage, and dispensed into a flat-bottom 96-well plate (Thermo Fisher, Nunc plates) with normal growth medium. Formation of viable colonies was assessed visually daily with a phase-contrast microscope from day 7 to day 14 post sorting. At the 2-week mark, clones were transferred to six-well plates, and grown to confluence to be frozen for future experiments and prepared for sequencing.
Low-pass WGS of clones
A total of 27 MCF10A cell pellets (18 clones deriving from micronucleated cells, nine control clones) and 11 RPE-1 cell pellets (11 clones deriving from micronucleated cells) were subjected to bulk-cell low-pass Illumina sequencing (NextSeq2000, P3, 100 bp paired-end sequencing) at EMBL’s Genomics Core Facility, to an approximate genomic coverage of 1× for screening purposes. Reads were aligned to the GRCh38 genome reference with BWA75, and read depth based CA calling was determined with support of the Control-FREEC tool76. A single case of a potential complex CAs was inferred on the basis of the chromosomal clustering of CAs inferred by read depth analysis.
Long-read WGS of clone 7
Clone 7 was re-established to obtain 10 × 106 cells for Oxford Nanopore Technologies (ONT) long-read sequencing. The library was prepared using the SQK-LSK114 ligation kit, and sequencing performed on PromethION flow-cells. The obtained coverage was 16×, and the reads showed an estimated N50 of 13.97 kb. Reads were aligned to the GRCh38 genome reference with minimap2 (ref. 77). Structural variant calling was performed with Sniffles78 and Delly79, and calls were curated manually to exclude false positives. Read depth profiles for the micronucleated clone 7 were generated using delly (cnv subcommand) with a window size of 25 kb and the standard GRCh38 mappability map. The read depth signal was segmented using the DNAcopy Bioconductor package. Somatic structural variants were called using sniffles2 and delly (lr subcommand). For both delly and sniffles2, we used another clone of MCF10A as a control to filter for somatic variants in the micronucleated clone 7. Subsequently, only candidate somatic structural variants called by both methods and larger than 10 kb were used. Single-nucleotide variants, as well as small insertions and deletions (indels), were called using Clair3. Haplotype phasing of the ONT reads was performed with WhatsHap to generate read depth plots by haplotype80. Telogator81 was used for telomere length inference from ONT reads generated from a MCF10A-derived clone (‘clone 7’), using the suggested ‘-r ont’ parameter recommended for handling Nanopore reads.
Modelling de novo CA rates
We developed an agent-based model82 to simulate CA acquisition in a growing population of cells, considering mitotic errors and micronuclei generation. During the simulation, cell agents are allowed to move between the states depicted in Fig. 3g. The probability Pij of transitioning from state i to state j is derived from long-term live-cell imaging experiments. Each cell agent is designed to possess three main attributes: cell cycle status, micronucleus status and CA status. The micronucleus status captures whether the cell possesses a micronucleus or not. The cell cycle status keeps track of an internal clock that simulates advancing through cell cycle until mitosis. Cell cycle duration is set at the median cell cycle duration measured in imaging experiments. The CA status captures whether the cell possesses a de novo CA. When the internal cell cycle clock reaches the end, mitosis or arrest occurs: the cell agent can move from interphase to a mitosis state (normal, laggard or bridge) or arrest. To simulate cell division, the current agent is moved to the arrest state and two new cells are generated and assigned to state 1 or 5, according to the transition probability associated with that specific mitosis type. Moreover, during mitosis, each cell has the possibility of acquiring a de novo CA according to the assigned rate R. Arrested cells are then removed from the simulation. Each simulation is initiated with an initial population of 50 cells and is stopped when the population reaches size 50,000, as we found empirically that the micronuclei and CA frequency usually stabilize by this time. Encouragingly, despite not being programmed explicitly into the model, the frequency of micronuclei stabilized at 5.0% and 38.3% for wild-type and TP53−/− cells respectively, closely mirroring our empirical data (Supplementary Fig. 6a,b). At the end of the simulation, we compute the sum of squared error between the simulated and target de novo CA frequencies. Details on how bound-constrained minimization was used to the CA rate estimation are the Supplementary Methods.
Fluorescent in situ hybridization
MCF10A cells were seeded on coverglass slides, subjected to targeted DSB induction, and allowed to recover for 48 h. Metaphase spreads were then prepared in situ directly on coverslips, as described elsewhere83. Sub-centromeric probes for Chr. 7 p and q arms were purchased from KromaTiD (Biocat catalogue no.: CEP-0013-C-KTD, CEP-0014-A-KTD). FISH was performed according to manufacturer instructions. After post-hybridization washes, DNA was stained with Hoechst 33342 and slides were mounted in anti-fade medium (Vectashield, Vector Laboratories). FISH images were acquired on a LSM 900 confocal microscope (Zeiss) at ×40 magnification and signals were evaluated visually.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.