Mouse procedures
NPp53 mice were maintained on a mixed C57BL/6-129Sv background and have been previously described7. Tamoxifen induction was performed in mice at 3–5 months of age by oral delivery of tamoxifen (MilliporeSigma; 100 mg kg–1 day–1 in corn oil) for 4 consecutive days as previously described58. The survival time of tumour-bearing mice in this study ranged from 228 to 435 days after tamoxifen induction (Supplementary Table 1). Mice were housed under specific pathogen-free conditions in individually ventilated autoclaved cages with irradiated feed and automated reverse-osmosis watering, under a 12-h dark–12-h light cycle with temperatures at 20–26 °C and humidity between 30 and 70%. All procedures followed protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Columbia University Irving Medical Center.
Establishment and maintenance of mouse prostate organoids
Tumour tissues from NPp53 mice were cut into two pieces, with half fixed in 10% formalin for paraffin embedding and the other half used for organoid establishment. Tissues were minced with scissors in 0.2% collagenase IV (Thermo Fisher Scientific, 17104019) and incubated at 37 °C for 30 min, followed by neutralization with 1:10 Hank’s buffer (Stemcell Technologies, 37150) supplemented with 10 µM Y-27632 (Stemcell Technologies) and 5% charcoal-stripped fetal bovine serum (CS-FBS; Gemini, 100-119). After centrifugation at 1,000 rpm for 10 min, pellets were incubated with prewarmed TrypLE (Thermo Fisher Scientific, 12605010) at 37 °C for 10 min. The cell suspension was then neutralized 1:10 with PBS, passed through a 100-µm cell strainer (Corning, 352360) and spun down at 1,000 rpm for 10 min. Before plating, cell numbers were counted in a TC20 automated cell counter (Bio-Rad).
Cells were resuspended in organoid culture medium supplemented with 10 µM Y-27632, 10 µM A83-01 (Tocris 2939) and 5% Matrigel (Corning 354234) and plated at a seeding density of approximately 50,000 cells per well in 96-well ultralow attachment microplates (Corning 3474). Organoid culture medium consisted of hepatocyte culture medium (Corning, 355056), 5% CS-FBS, 1× GlutaMAX supplement (Thermo Fisher Scientific, 35050061), 5 ng ml–1 EGF, 100 µg ml–1 primocin (Invivogen, ant-pm-1) and 100 nM DHT, as previously described59. For heterogeneous neuroendocrine organoids, such as NPPO-1 and NPPO-5, organoid culture medium was replaced every 4 days. For maintenance of homogeneous neuroendocrine organoids, such as NPPO-1NE, NPPO-2, NPPO-4 and NPPO-6, we used neuroendocrine organoid culture medium, which was identical to the organoid culture medium except that no EGF was added; the medium was replaced every 4 days. For maintenance of non-neuroendocrine NPPO organoids (NPPO-1nonNE, NPPO-7, NPPO8 and NPPO9), we used the standard organoid culture medium. In organoid experiments involving CRISPR–Cas9-mediated targeting of Nsd2, oncohistone H3.3K36M expression or NSD2i treatment, DHT was removed from the culture medium starting at day 0, and organoids were maintained in the absence of DHT for subsequent analyses.
For passaging, organoids were collected by centrifugation at 1,000 rpm for 1 min, followed by the addition of 1 ml pre-warmed TrypLE for 10 min at 37 °C for cell dissociation. After neutralization with 10 ml PBS, cells were spun down and counted, with approximately 50,000 cells plated per well in 96-well ultralow attachment microplates (Corning, 3474). To generate cryopreserved stocks, organoids were frozen in 90% CS-FBS and 10% DMSO and stored in liquid nitrogen. We considered neuroendocrine organoid lines to be successfully established when they could be stably passaged, cryopreserved and recovered without loss of neuroendocrine phenotypes. Details regarding establishment of the TKO organoids (Extended Data Fig. 4a) from Ptenfl/fl;Rb1fl/fl;Trp53fl/fl;mT:mG (PtRP) prostate epithelial cells are described in a separate manuscript (in preparation). Organoid cultures routinely tested negative for mycoplasma contamination.
Human prostate tumour organoids
MSKPCa2, MSKPCa10, MSKPCa14, WCM154 and WCM1262 organoids have been previously described5,36,37. Human prostate tumour organoids were maintained in 80% Matrigel in human neuroendocrine culture medium, which was replaced every other day. Human neuroendocrine culture medium consisted of human hepatocyte culture medium (LifeNet Health LifeSciences, MED-HHCM-500ML), human hepatocyte culture medium supplement (LifeNet Health LifeSciences, MED-HHCMS), 5% CS-FBS, 1× GlutaMAX, 5 ng ml–1 EGF (Thermo Fisher Scientific, PHG0311), 100 µg ml–1 primocin and 10 nM DHT.
H&E staining
For tissue processing and embedding, organoids were fixed in 10% formalin (Fisher Scientific, SF100-4) for 1 h, washed once with PBS, placed in rat tail collagen I (Corning, 354249) and incubated at 37 °C for 30 min. The collagen button was then put into a biopsy cassette (Fisher Scientific, 15182705E) and fixed in 10% formalin for 24 h. After replacing the formalin with 70% ethanol, the cassettes were put into an automated tissue processor for tissue processing and embedding.
Paraffin-embedded blocks were sectioned into 5-µm sections using a microtome and dried onto microscope slides at room temperature. Paraffin sections were baked at 65 °C for 15 min before deparaffinization with 3 changes of xylene (5 min each). The slides were hydrated through 100%, 95% and 95% ethanol, 5 min each, rinsed in tap water for 2 min and incubated in Gill Hematoxylin 3 (Epredia, 72611) for 3–30 min. Slides were rinsed in tap water and dipped 3–5 times in 0.5% acid alcohol (Leica Biosystems, 3803651), followed by rinsing in tap water and bluing with Scott’s Tap Water (Electron Microscopy Sciences, 2607007) for 5 min. After rinsing in tap water, slides were incubated in 95% ethanol for 5 min and counterstained with eosin (StatLab, S1761GL) for 1–3 min. Slides were passed through 70%, 95% and 100% (3 times) ethanol, rinsed 3 times in xylene and coverslipped with mounting medium (StatLab, MMC0126). Images were captured using an Olympus BX 61 VS slide scanner, and image acquisition was performed using Olympus VS-ASW (v.2.5) software.
Immunofluorescence staining
Paraffin sections (5 µm) were dried onto microscope slides at room temperature, incubated at 65 °C for 15 min before deparaffinization through 3 changes of xylene (5 min each), hydrated in 100%, 95%, 95% and 75% ethanol (5 min each) and washed in tap water for 2 min. Antigen retrieval was performed through immersion in boiling citrate buffer (pH 6) for 10 min, cooling to room temperature for 30 min and incubation in Milli-Q water at room temperature for 10 min. Sections were permeabilized with 0.5% Triton X-100 in PBS (MilliporeSigma, 11332481001) for 10 min and blocked in 10% goat serum (Thermo Fisher Scientific, 50062Z) for 1 h. Primary antibodies at the indicated dilutions (Supplementary Table 6) were added to sections and incubated overnight at 4 °C. The next day, sections were washed with PBS 3 times, 15 min each, and incubated with secondary antibodies at room temperature for 1 h. After washing with PBS 3 times, 15 min each, nuclei were stained with DAPI (Thermo Fisher Scientific D1306) for 5 min. Slides were washed with PBS and mounted with Vectashield Antifade mounting medium (Vector Laboratories H-1200-10). Images were captured using a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems) using Leica Application Suite Advanced Fluorescence (LAS AF v.2.6.0) software.
scRNA-seq
For scRNA-seq, NPPO-1, NPPO-2, NPPO-4, NPPO-5 and NPPO-6 organoids were analysed at passage 2. Organoids were dissociated by incubation with pre-warmed TrypLE at 37 °C for 10 min, neutralization with 1:10 5% CS-FBS–PBS, centrifugation at 1,000 rpm for 1 min and filtration 3 times through a 40-µm cell strainer (Corning 431750). Cells were spun at 1,000 rpm for 5 min, pellets were dissociated with 5% CS-FBS–PBS and resuspended at 1,000 cells per µl after counting using a Countess II FL automated cell counter. Libraries were prepared using a Chromium Next GEM Single Cell 3′ Reagent kit (v3.1) by the Columbia University Single Cell Analysis Core. Approximately 5,000 cells were loaded onto a Chromium Controller (10x Genomics) to generate gel beads-in-emulsion (GEMs), and barcoded, full-length cDNA from poly-adenylated mRNA was generated and amplified by PCR. Chromium Gene Expression libraries were prepared for paired-end sequencing, and scRNA-seq data were processed using Cell Ranger software (10x Genomics, v.2.1.1 for MJ002 and MJ004; v.3.0.2 for MJ005, MJ007, MJ008 and MJ012; v.5.0.1 for MJ014 and MJ015; see Supplementary Table 3 for sample identities) by the Columbia University Single Cell Analysis Core. Quality control metrics are provided in Supplementary Table 7.
Single-nucleus ATAC–seq
NPPO-1 organoids at passage 8 were dissociated using TrypLE, passed through a 40-µm cell strainer 3 times, and cell numbers quantified using an automated cell counter. Approximately 1 × 106 cells in 0.04% BSA–PBS (Miltenyi Biotec 130-091-376) were used for single nucleus isolation. Cells were spun at 1,000 rpm for 5 min at 4 °C, dissociated for 5 min with 100 µl ice-cold lysis buffer, neutralized with 1 ml chilled wash buffer, spun at 1,000 rpm for 5 min at 4 °C and resuspended in chilled nuclei buffer included in a Chromium Single Cell ATAC Library kit (10x Genomics). Wash buffer contained 10 mM Tris-HCl (pH 7.4), 10 mM NaCl, 3 mM MgCl2, 1% BAS and 0.1% Tween-20 in nuclease-free water. Lysis buffer contained 10 mM Tris-HCl (pH 7.4), 10 mM NaCl, 3 mM MgCl2, 0.1% Tween-20, 0.1% Nonidet P40 substitute, 0.01% digitonin and 1% BAS in nuclease-free water. Nucleus concentration was determined using a Countess II FL automated cell counter. Approximately 5,000 nuclei were loaded onto a Chromium Controller at the Columbia University Single Cell Analysis Core. Single-nucleus ATAC–seq libraries were prepared following the manufacturer’s instructions (Chromium Next GEM Single Cell ATAC Reagent Kits v.1.1, 10X Genomics). In brief, nuclei were transposed and partitioned into GEMs. 10x barcodes were added to index the transposed DNA of each individual nucleus. Libraries were generated by PCR and sequenced on an Illumina NextSeq 550 platform. Paired-end sequencing data were processed with Cell Ranger ATAC (v.1.0.1) by the Columbia University Single Cell Analysis Core. Quality control metrics are provided in Supplementary Table 7.
Isolation of neuroendocrine and non-neuroendocrine cells from NPPO-1 organoids
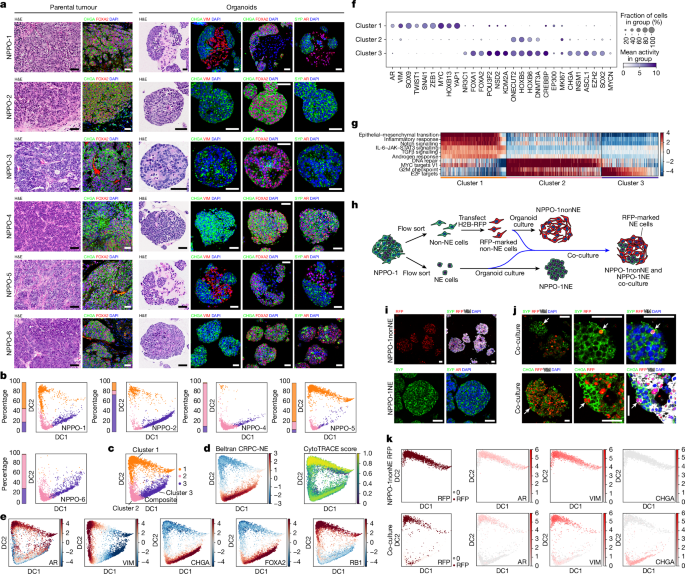
To sort neuroendocrine and non-neuroendocrine populations, NPPO-1 organoids at passage 2 were incubated with prewarmed TrypLE at 37 °C for 10 min, neutralized with 1:10 PBS and 5% CS-FBS, spun at 1,000 rpm for 1 min, resuspended with PBS and dissociated into single cells by gentle pipetting. The cells were filtered 3 times through a 40-µm cell strainer (Corning, 431750), spun at 1,000 rpm for 5 min and resuspended with PBS and 2% CS-FBS. After filtering through a Falcon tube with a 35-µm strainer cap (Corning 352235), cell numbers were counted in a TC20 automated cell counter, and the volume was adjusted to a final cell concentration of 5,000 cells per µl.
Flow sorting was performed on an BD Influx cell sorter (BD Biosciences, X64650000124) at the Flow Cytometry Core of the Columbia Center for Translational Immunology. Gating by forward scatter (FSC) and side scatter (SSC) was used to exclude debris, and doublets were excluded by gating on trigger pulse width against FSC height. Individual neuroendocrine and non-neuroendocrine tumour cells were sorted based on scatter parameters. Neuroendocrine tumour cells have less internal complexity (granularity) than non-neuroendocrine tumour cells and exhibit lower intensity SSC. Flow sorting data were collected and analysed using BD FACS software (BD Biosciences, v.1.2.0.142). Cell purity was assessed after flow sorting by scRNA-seq.
Lineage tracing in organoids
Flow-sorted neuroendocrine cells from NPPO-1 organoids were maintained in neuroendocrine organoid culture medium with 5% Matrigel. Half of the flow-sorted non-neuroendocrine cells were used for scRNA-seq (Columbia University Single Cell Analysis Core) immediately after sorting. The other non-neuroendocrine cells were transfected with H2B-RFP (Addgene, 26001) lentivirus. Approximately 70% of non-neuroendocrine cells were labelled with H2B-RFP at 3 days after transfection, in the absence of antibiotic selection. On day 7, the cells were digested with TrypLE and passed 3 times through a 40-µm cell strainer to ensure a single-cell suspension, followed by cell counting. For co-culture, H2B-RFP-labelled non-neuroendocrine cells were seeded together with neuroendocrine cells at a ratio of 2:3 in 96-well ultralow attachment microplates; as a control, H2B-RFP-labelled non-neuroendocrine cells were seeded alone. The resulting organoids were cultured in organoid culture medium with 5% Matrigel and analysed at passage 4 by immunostaining and scRNA-seq.
Imaging of histone and DNA modifications
To screen for differential expression of histone modifications between neuroendocrine and non-neuroendocrine tumour cells in NPPO-1 organoids, we performed immunofluorescence staining with antibodies that detect the neuroendocrine markers SYP or CHGA, the non-neuroendocrine marker VIM, and various histone and DNA modifications (Supplementary Table 6). Images were captured using a Leica TCS SP5 confocal laser scanning microscope, and images were acquired using Leica Application Suite Advanced Fluorescence software (LAS AF v.2.6.0). Fluorescence intensity was measured using ImageJ (NIH; v.1.52K) using three parameters: area, integrated density (IntDen) and mean grey value. The background was measured from a region that had no fluorescence on the same image. Intensity was calculated using the formula: Intensity = IntDen – (area \(\times \) mean fluorescence of background readings). Measurements were collected from three independent organoids, and results were plotted using Prism 9 (GraphPad software, v.10.5.0). Unpaired t-tests were used to compare means, and P values were calculated from two-tailed t-tests.
Analysis of NSD2 expression in human datasets
To evaluate differences in gene expression of NSD1, NSD2 and NSD3 in human prostate tumour samples, we analysed bulk RNA-seq from 49 patients with CRPC (15 CRPC-NE and 34 CRPC-Adeno) from a published dataset20. For each gene, P values were calculated using two-tailed t-tests comparing the mean log-transformed counts per million (CPM) between the CRPC-NE and CRPC-Adeno groups.
We also analysed a published scRNA-seq dataset25. Expression data from tumour cells were downloaded from the Gene Expression Omnibus (GEO accession GSE264573) and supplementary file ‘msk.integrated.remove.cellcycle.tumor.cells’. We used the same annotation for NEPC, CRPC and treatment-naive/CSPC, as released in supplementary file ‘pnas.2322203121.sd03.xlsx’. Violin plots were generated using the seaborn Python library. For each gene, violin plots were created to visualize the distribution of gene expression across three subtypes. Group means were overlaid as horizontal lines to aid visual interpretation. Pairwise statistical comparisons between subtypes were performed using nonparametric Mann–Whitney U-tests (two-sided), implemented using the statannotations package.
Histone extraction and western blotting
Histone extract lysates were prepared by acid extraction as previously described60. In brief, cells were resuspended in hypotonic lysis buffer (10 mM Tris-Cl pH 8.0, 1 mM KCl, 1.5 mM MgCl2 and protease inhibitor cocktail (MilliporeSigma, 11697498001)) and incubated on a rotator for 30 min at 4 °C. Nuclei were isolated by centrifugation (16,000g for 10 min at 4 °C) and resuspended in H2SO4. After overnight incubation on a rotator at 4 °C, debris was removed by centrifugation (16,000g for 10 min at 4 °C) and histones were precipitated from the supernatant using TCA (MilliporeSigma, T6399). Purified histones were washed with cold acetone and resuspended in H2O. Samples were quantified using a Bradford assay (Bio-Rad, 5000205), and protein lysates were prepared using SDS lysis buffer (Thermo Fisher Scientific, LC2676).
For western blotting, whole-cell lysates were prepared by resuspending cells in SDS lysis buffer, sonicating for 10 s twice and boiling for 8 min. Protein lysates were resolved on 3–8% (Thermo Fisher Scientific, EA0375BOX) or 4–12% gradient SDS–PAGE gels (Thermo Fisher Scientific, NP0321BOX), transferred to nitrocellulose membrane, blocked in 5% non-fat milk in PBS plus 0.5% Tween-20, probed with primary antibodies at the indicated dilutions (Supplementary Table 6) and detected with horseradish-peroxidase-linked anti-rabbit IgG (Cell Signaling Technology, 7074) or anti-mouse IgG (Cell Signaling Technology, 7076). Blots were imaged using a ChemiDoc MP imaging system (Bio-Rad, 17001402) or exposed to X-ray film (Research Products International, 248300). For gel source data, see Supplementary Fig. 1.
Bulk RNA-seq
Organoids were dissociated into single cells using TrypLE, and total RNA was isolated using a MagMAX-96 for Microarrays Total RNA Isolation kit (Thermo Fisher Scientific, AM1839). In brief, cells were lysed in 1 ml TRI reagent per 5 million cells and incubated for 5 min at room temperature. The homogenate was mixed with 0.1 volume 1-bromo-3-chloropropane (MilliporeSigma, B9673), incubated at room temperature for 5 min and centrifuged at 12,000g for 10 min at 4 °C. Next, 100 μl of the aqueous phase was transferred to an 8-strip EpiCypher tube (DNase/RNase-free), and 50 μl of 100% isopropanol (MilliporeSigma I9516) was added with shaking for 1 min. Next, 10 μl RNA binding beads was added, followed by shaking for 3 min. The RNA binding beads were then captured on a magnetic stand and the beads were washed twice with 150 μl wash solution. The beads were dried for 2 min, and RNA was eluted in 50 μl elution buffer. All samples were DNase-treated before library construction. Quality assessment was performed using a 4200 TapeStation system (Agilent), with a median RNA integrity number of 9.2 (range of 8.7–9.8). Libraries were generated using the Illumina Stranded mRNA Prep, and 150-bp paired-end sequencing was performed to a minimum of 20 million reads per sample on an Illumina HiSeq platform.
CUT&Tag
Before cell preparation, 10 µl per sample concanavalin A (ConA)-coated magnetic beads (Bangs Laboratories BP531) was transferred into a 1.5-ml LoBind tube (Eppendorf 022431021) and mixed by gentle vortexing with 0.4 ml binding buffer (20 mM HEPES (pH 7.5; Thermo Fisher Scientific, 15630080), 10 mM KCl (MilliporeSigma, 60142), 1 mM CaCl2 (MilliporeSigma, 21115) and 1 mM MnCl2 (MilliporeSigma M1787) in nuclease-free water). The tubes were placed on a magnetic stand (Thermo Fisher Scientific, 12321D) to clear and remove liquid, with the wash repeated once with binding buffer.
For experiments requiring normalization of input DNA, 10,000 Drosophila Schneider 2 (S2) cells (Thermo Fisher Scientific, R69007) were combined with 200,000 experimental cells at a ratio of 1:20. S2 cells were maintained in Schneider’s Drosophila medium (Thermo Fisher Scientific, 21720024), 10% heat-inactivated FBS (Gemini, 100-106) and 100 µg ml–1 primocin. S2 cells were plated in 96-well ultralow attachment microplates at a seeding density of approximately 100,000 cells per well and maintained at 28 °C without CO2.
Organoids were dissociated into single cells using TrypLE, counted using a TC20 automated cell counter, and 200,000 cells per sample were used for profiling. After centrifugation at 1,000 rpm at room temperature, cell pellets were washed once with 1 ml wash buffer (20 mM HEPES (pH 7.5), 150 mM NaCl, 0.5 mM spermidine (MilliporeSigma, S2501) and cOmplete, EDTA-free protease inhibitor cocktail (MilliporeSigma, 11873580001) in nuclease-free water). Cell pellets were resuspended with 10 µl ConA beads in 0.4 ml wash buffer and incubated for 10 min at room temperature. The tubes were then placed on a magnetic stand (New England Biolabs, S1515S) to clear and remove the liquid, with pellets resuspended with 50 µl ice-cold Dig-wash buffer (wash buffer with 0.05% digitonin) and placed on ice for 10 min to lyse the cells. The samples were then transferred to an 8-strip tube (EpiCypher, 10-0009). Each sample was incubated with 0.5 µg primary or IgG control antibody (Supplementary Table 6) and placed on a nutator at 4 °C overnight. The next day, samples were incubated with 1 µl secondary antibody in 50 µl ice-cold Dig-wash buffer on a nutator at room temperature for 1 h. After washing with Dig-Wash buffer 3 times, beads were resuspended with 50 µl Dig-300 buffer (20 mM HEPES (pH 7.5), 300 mM NaCl, 0.5 mM spermidine, 0.01% digitonin, cOmplete, EDTA-free protease inhibitor cocktail in nuclease-free water) containing 2.5 µl pA-Tn5 adapter complex (EpiCypher, 15-1017) and placed on a nutator at room temperature for 1 h. After washing 3 times with Dig-300 buffer, the beads were resuspended in 150 µl Tagmentation buffer (Dig-300 buffer with 10 mM MgCl2) and incubated at 37 °C for 1 h.
To stop tagmentation and to solubilize DNA fragments, 5 µl 0.5 M EDTA (Thermo Fisher Scientific, AM9260G), 1.5 µl 10% SDS (MilliporeSigma, 71736) and 1.25 µl 20 mg ml–1 proteinase K (Thermo Fisher Scientific, EO0491) was added to each sample and incubated at 37 °C overnight. The next day, phenol–chloroform–isoamyl alcohol (25:24:1, v/v; Thermo Fisher Scientific, 15593049) was used to extract nucleic acids. DNA was precipitated with 100% ethanol (MilliporeSigma, E7023) and dissolved in 25 μl RNase-free water. To remove RNA contamination before library preparation, DNA samples were incubated with 25 µg ml–1 RNase A (Thermo Fisher Scientific, EN0531) at 37 °C for 10 min. Library DNAs were amplified by PCR using Illumina universal i5 primers, Nextera barcoded i7 primers (Supplementary Table 8) and NEBNext HiFi 2× PCR master mix (New England Biolabs, M0541). The PCR conditions were as follows: cycle 1: 72 °C for 5 min; cycle 2: 98 °C for 30 s; cycle 3: 98 °C for 10 s; cycle 4: 63 °C for 10 s, repeating 14 times, followed by 72 °C for 1 min and hold at 8 °C. Post-PCR clean-up was performed using 1:1 volume Ampure XP beads (Beckman Coulter, A63880). Samples were dissolved in 10 mM Tris-HCl (pH 8; MilliporeSigma, T2694) for sequencing. Size distribution and concentration of libraries were determined by capillary electrophoresis using a 4200 TapeStation (Agilent). Paired-end sequencing (2 × 150 bp) was performed on pooled libraries, with 10–12 million reads per library, using an Illumina HiSeq system. Quality control metrics are provided in Supplementary Table 9 and Supplementary Fig. 2.
CUT&Tag data analysis
CUT&Tag reads were trimmed using cutadapt (v.3.6) and aligned to the mouse reference genome (mm10) and Drosophila (BDGP6) using BOWTIE2 (v.2.4.2) with the following options: –very-sensitive-local –no-unal –no-mixed –no-discordant –phred33 -I 10 -X 700. Scaling factors for spike-in normalization were determined by the ratio between the number of reads aligned to the mouse genome and the Drosophila genome. Potential PCR duplicates were removed by the markdup function of sambamba (v.1.0.1). Peak calling was performed using SEACR (v.1.3) or MACS2 (v.2.2.8) with IgG input as control. Intersect function of bedtools (v.2.27.1) was used to identify H3K27ac enhancers corresponding to H3K27ac peaks not overlapping promoters and found in broader H3K36me2 domains. Motif analysis was performed using the simple enrichment analysis function from MEME suit (v.5.5.7) with the JASPAR 2022 Core motif database61. Genomic enrichment of CUT&Tag signals for each histone modification was analysed using deeptools (v.3.5.5) and visualized using IGV (v.2.13.0). Coverage tracks were generated using the bamCoverage function of DeepTools with the bin size of 50 bp, with problematic ENCODE regions (the ENCODE blacklist for mm1062) and amplified genomic regions blacklisted and normalized using the appropriate scaling factor (mapped Drosophila reads). Heatmap and enrichment plots of H3K36me2, H3K27me3, H3K36me3 or H3K27ac over H3K36me2 or H3K27ac peaks were generated using the computeMatrix (reference- point, – a 5000 – b 5000) and plotHeatmap functions implemented in DeepTools.
RNA-seq reads were aligned to the mouse reference genome (mm10) using HISAT2 (v.2.1.0). The mapped reads count of each gene was measured by featureCounts (v.1.6.1). The RNA-seq read count matrix was combined with the CUT&Tag signal read count matrix for all gene loci in R (v.4.1.2).
Lentivirus production and transfection
Lentiviruses were generated by the transfection of 293T cells with the indicated expression plasmid and the psPAX2 (Addgene, 12260) and pVSV.G (Addgene, 14888) packaging vectors at a ratio of 4:2:3, respectively. Viral supernatants were collected at 48, 72 and 96 h after transfection, filtered and concentrated using a Lenti-X Concentrator (Takara Bio, 631232). For CRISPR–Cas9-mediated gene knockout, we used the lentiCas9-blast plasmid (Addgene, 52962) and a custom vector for sgRNA (U6-sgRNA-EFS-Puro-P2A-TurboRFP in a pLL3-based lentiviral backbone; gift from S. Lowe). For sgRNA design, the CRISPick platform (BROAD institute) was used. HA-tagged H3.3K36M was overexpressed in the pCDH vector (gift from D. Allis). The following sgRNAs were used in the experiment: sgControl, 5′ GAG ATA AGC ATT ATA ATT CCT 3′; sgNsd2 (mouse): 5′ TCA GGG TCT CAC AAT TGG GC 3′; sgNSD2 (human): 5′ GCA CCA GCT CAC GTT GAC GT 3′.
For transfection, organoids were incubated with high-titre lentivirus in culture medium supplemented with 8 µg ml–1 polybrene (MilliporeSigma, TR-1003). Medium containing virus was removed on the next day and switched to normal organoid medium with Matrigel. Selection with appropriate antibiotics was performed at 3 days after transfection for 7–14 days.
Multiplexed staining of TMAs
Primary antibodies were tested on prostate tumour samples to verify the expected pattern of staining and were titrated at four concentrations to determine the best signal-to-noise ratio. Multiplexed staining was performed using an Opal 6-plex detection kit (Akoya Biosciences, NEL871001KT) on a Bond Rx Research Stainer (Leica Biosystems), adding DAPI as a nuclear marker. Slides were imaged using a Vectra Polaris Automated Quantitative Pathology Multispectral Imaging system (Akoya Biosciences). Exposure times were optimized under the constraint that no pixel saturated the detector. These studies were conducted under protocols approved by Weill Cornell Medical Center. All patients with prostate cancer or their families provided informed consent for research use of biospecimens and clinical data under an institutional approved protocol (IRB #1008011210).
Analysis of patient survival curves
To evaluate the association of NSD2 with overall survival in mCRPC, two independent mCRPC biopsy RNA-seq cohorts were used: (1) a cohort of 159 mCRPC transcriptomes generated by the PCF–SU2C Prostate Cancer Dream Team13 (141 mCRPC transcriptomes from this dataset were used for the survival analyses as survival data were not available for 18 patients); (2) a cohort of 95 mCRPC transcriptomes from patients treated at the RMH31 were analysed (94 mCRPC transcriptomes were used for the survival analyses as survival data were not available for 1 patient). Transcriptomes were aligned to the human reference genome (GRCh37/hg19) using TopHat2 (v.2.0.7). Gene expression as fragments per kilobase of transcript per million mapped reads (FPKM) was calculated using Cufflinks (v.2.2.1). Kaplan–Meier studies evaluated overall survival outcomes. To examine the correlation of NSD2 expression with neuroendocrine gene expression, a 29 gene neuroendocrine signature20 was used to calculate an accumulated signature score for each sample by summing the z score of the signature genes.
Computational analysis of multiplex images
All TMA cores underwent post-acquisition processing by linear spectral unmixing and deconvolved using InForm software (Akoya Biosciences, v.3.1), and the tiles were stitched using Halo (Indica Labs, v.3.6). Tissue segmentation of the images was performed using a deep-learning classifier by training the algorithm ‘DenseNet V2’ from the Halo AI plug-in (Indica Labs, v.3.6), using only the DAPI channel as information for the training. Eight different classes were defined for both tissue segmentation and quality control issues: background, stroma, malignant tumour, benign glands, necrosis, liver tissue, decalcified tissue and out-of-focus regions. The performance of the classifier was evaluated by a pathologist (F.S.) to ensure that the majority of tissue compartments were properly classified. Cell segmentation was performed using a pre-trained deep-learning model already present in Halo AI (‘nuclei seg’) and applied to the DAPI channel only. Using the module ‘analysis’ in the Halo software, all the biomarkers were then quantified using the segmentation algorithms to generate a counts matrix representing the average expression of each marker in each cell. Thresholding for each marker was performed using a Gaussian mixture model of statistically robust cut-off values for low versus high intensity of the markers.
The x and y coordinates for the precise nuclei or cytoplasmic marker locations from the immunofluorescence intensities were mapped for each TMA core. The coordinate point location was taken for multiple-localization analysis in different classes of markers. There are Mn classes of markers, for which each class is composed of a different combination of markers. For any given combination of markers, M1, …, Mn (each composed of nuclei or cytoplasm as detected with thresholds on their intensities), we classified a given nucleus or cytoplasm NCi coordinate, k in [1, …, i], belonging to M1, …, Mn class. We repeated this process for each NCi in M1, …, Mn to form a distribution of multiple localizations in the different classes of markers, Ci. Measures for Ci were recorded for each TMA core sample and normalized using the total number of NCi belonging to M1, …, Mn class per patient. For any given marker, M1, …, Mn, we also measured the overall distribution at the single-localization level. The mean value for the measures was implemented by Welch ANOVA or unpaired t-test (two-tailed) in Prism (GraphPad software, v.10.5.0).
Flow sorting of transfected cells
For CRISPR–Cas9-mediated gene knockout experiments, organoids were transfected with the lentiCas9-blast plasmid and blasticidin selection to establish stable lines. A custom vector for the sgRNA lentivirus carrying a TurboRFP reporter and puromycin antibiotic was then transfected into the Cas9-expressing organoids. After 14 days of antibiotic selection, we performed FACS on the PE channel to sort RFP+ cells on an BD Influx cell sorter (BD Biosciences, X64650000124), as described above. Approximately 1 × 106 RFP+ cells were collected in 0.04% BSA in PBS (Miltenyi Biotec, 130-091-376) for single-nucleus isolation and multiome ATAC–seq (10x Genomics). Additional collected RFP+ cells were used for organoid culture.
For experiments in which mouse neuroendocrine tumour organoids were transfected with an HA-tagged H3.3K36M lentiviral vector, we performed flow cytometry at 14 days after antibiotic selection to determine the purity of the culture. Organoids were dissociated into single cells and resuspended in 100 µl 4% paraformaldehyde (1 × 106 cells) to fix for 15 min at room temperature. The fixed cells were neutralized with 1 ml PBS, washed once with PBS and resuspended in 0.5 ml PBS. The cells were permeabilized for 10 min by the addition of 0.5 ml 1% Triton-X100 with gentle vortexing to a final concentration of 0.5% Triton-X100. Cells were washed in 10 ml PBS and resuspended with 100 µl Cy5.5-conjugated mouse anti-HA-Tag antibody (clone 6E2, Cell Signaling Technology, 62145) diluted 1:50 in 0.5% BSA in PBS. Cells were incubated with antibody for 1 h in the dark at room temperature, washed twice in 0.5% BSA, resuspended in 300 µl 0.5% BSA and filtered through a Falcon tube with a 35-µm cell strainer cap. Flow cytometry was performed on the APC channel using a FACSCanto II flow cytometer (BD Bioscience) as described above, using Cy5.5+ events to determine the percentage of HA-Tag+ cells. Flow data were analysed using FlowJo (BD, v.10.8.2). The same batch of cells was collected in 0.04% BSA in PBS for single-nucleus isolation and multiome single-nucleus ATAC–seq and snRNA-seq.
Single-nucleus multiome ATAC–seq and RNA-seq
Organoids were digested into single cells using TrypLE, passed through a 40-µm cell strainer 3 times, and cell numbers were quantified using an automated cell counter. Approximately 1 × 106 cells in 0.04% BSA–PBS (Miltenyi Biotec, 130-091-376) were used for single-nucleus isolation. Cells were spun at 1,000 rpm for 5 min at 4 °C, dissociated for 5 min with 100 µl ice-cold lysis buffer, neutralized with 1 ml wash buffer, spun at 1,000 rpm for 5 min at 4 °C and resuspended in chilled nuclei buffer included in the Single Cell Multiome ATAC kit (10x Genomics). Wash buffer contained 10 mM Tris-HCl (pH 7.4), 10 mM NaCl, 3 mM MgCl2, 1% BAS, 0.1% Tween-20, 1 mM DTT (MilliporeSigma, 646563) and 1 U µl–1 RNase inhibitor (MilliporeSigma, 3335399001) in nuclease-free water. Lysis buffer contained 10 mM Tris-HCl (pH 7.4), 10 mM NaCl, 3 mM MgCl2, 1% BAS, 0.1% Tween-20, 0.1% Nonidet P40 substitute, 0.01% digitonin and 1 mM DTT, 1 U µl–1 RNase inhibitor in nuclease-free water. Nucleus concentration was determined using a Countess II FL Automated cell counter.
Approximately 5,000 nuclei were loaded onto a Chromium X Controller (10x Genomics) at the Columbia University Single Cell Analysis Core. Single-cell multiome ATAC–seq and RNA-seq libraries were prepared following the manufacturer’s instructions (Chromium Next GEM Single Cell Multiome Reagent kit, 10x Genomics). In brief, nucleus suspensions were transposed and adapters added to the ends of the DNA fragments. Single Cell Multiome ATAC + GEX Gel Beads include a poly(dT) sequence that enables production of barcoded, full-length cDNA from poly-adenylated mRNA for the gene expression (GEX) library and a spacer sequence that enables barcode attachment to transposed DNA fragments for the ATAC library. The GEMs were generated by combining barcoded gel beads, transposed nuclei and a master mix. Barcoded transposed DNA and barcoded full-length cDNA from poly-adenylated mRNA were amplified by PCR. Single-cell multiome ATAC–seq and RNA-seq libraries were prepared for paired-end sequencing, and data were processed using Cell Ranger ARC (10x Genomics, v.1.0.0 for MJ018, MJ019, MJ020, MJ021, MJ022 and MJ023; v.2.0.2 for MJ024 and MJ025; see Supplementary Table 3 for sample identities) by the Columbia University Single Cell Analysis Core. Quality control metrics are provided in Supplementary Table 7.
Enzalutamide treatment of organoids
To generate drug–response curves, organoids were digested with TrypLE for 10 min at 37 °C, neutralized with PBS, gently dissociated into single cells and passed through a 100-µm cell strainer. Cells were resuspended in 5% Matrigel in neuroendocrine organoid culture medium lacking DHT and plated in triplicate at a seeding density of 5,000 cells per well in 96-well ultralow attachment microplates. The next day, 7 doses of enzalutamide in 0.1% DMSO were dispensed at 1.5-fold dilution from 1 µM to 11.25 µM. Cell viability was assayed after 5 days using CellTiter-Glo 3D (Promega G9683), and luminescence was measured using a GloMax Explorer multimode plate reader (Promega, v.3.1.0). Background luminescence was measured in medium without cells. The percentage of viable cells was calculated using the following formula:
$${\rm{V}}{\rm{i}}{\rm{a}}{\rm{b}}{\rm{l}}{\rm{e}}\,{\rm{c}}{\rm{e}}{\rm{l}}{\rm{l}}{\rm{s}}\,({\rm{ \% }})=\frac{{\rm{E}}{\rm{x}}{\rm{p}}{\rm{e}}{\rm{r}}{\rm{i}}{\rm{m}}{\rm{e}}{\rm{n}}{\rm{t}}{\rm{a}}{\rm{l}}\,{\rm{v}}{\rm{a}}{\rm{l}}{\rm{u}}{\rm{e}}-{\rm{b}}{\rm{a}}{\rm{c}}{\rm{k}}{\rm{g}}{\rm{r}}{\rm{o}}{\rm{u}}{\rm{n}}{\rm{d}}\,{\rm{r}}{\rm{e}}{\rm{a}}{\rm{d}}{\rm{i}}{\rm{n}}{\rm{g}}}{{\rm{V}}{\rm{e}}{\rm{h}}{\rm{i}}{\rm{c}}{\rm{l}}{\rm{e}}\,{\rm{v}}{\rm{a}}{\rm{l}}{\rm{u}}{\rm{e}}-{\rm{b}}{\rm{a}}{\rm{c}}{\rm{k}}{\rm{g}}{\rm{r}}{\rm{o}}{\rm{u}}{\rm{n}}{\rm{d}}\,{\rm{r}}{\rm{e}}{\rm{a}}{\rm{d}}{\rm{i}}{\rm{n}}{\rm{g}}}\times 100$$
Drug–response curves were generated by nonlinear regression using the percentage of viable cells against the logarithm of drug concentrations using Prism (GraphPad software, v.10.5.0). IC50 values were calculated by the equation log (inhibitor) versus response (variable slope, four parameters). Two-way ANOVA was used to compare dose–response curves.
Similar methods were used to determine response of mouse or human organoids to defined doses of enzalutamide, using 5,000 cells per well (mouse) or 10,000 cells per well (human). The percentage of viable cells from different treatment groups were plotted using Prism (GraphPad software, v.10.5.0). Unpaired t-tests were used to compare means between two groups. All experiments were repeated independently at least three times with consistent results observed.
Synthesis and analysis of NSD2i
To assess the consequences of pharmacological targeting of NSD2, we used a small molecule corresponding to compound 160 of US patent 2025/0276971 A1 (ref. 63) (Extended Data Fig. 9a), which is similar to KTX-1001, a compound currently being tested in an early-phase clinical trial for t(4,14) translocation-positive multiple myeloma (ClinicalTrials.gov identifier NCT05651932). We synthesized this compound using a previously described method63, with minor modifications (Extended Data Fig. 9b). Full details of the chemical synthesis and characterization are provided in the Supplementary Information. The purified NSD2i compound was then tested for its ability to inhibit the activity of a range of histone methyltransferases in vitro.
Expression and purification of recombinant methyltransferase proteins
NSD1(SET) (amino acids 1853–2093, NCBI sequence: NC_000005.10), NSD2(SET) (amino acids 958–1365, NCBI sequence: NC_000004.12), NSD3(SET) (amino acids 1021–1320, NCBI sequence: NC_000008.11), ASH1L(SET) (amino acids 1980–2564, NCBI sequence: NC_000001.11), SETD2(SET) (amino acids 1323–2564, NCBI sequence: NC_000003.12) and SUV39H1(SET) (amino acids 82–412, NCBI sequence: NC_000023.11) were cloned into pGEX-6P-1. Escherichia coli BL21 cells were transformed with the respective expression vectors and cultivated in LB medium (10 g l–1 tryptone, 5 g l–1 yeast extract and 10 g l–1 NaCl) supplemented with 0.1 mM isopropyl β-d-1-thiogalactopyranoside (MilliporeSigma) at 18 °C for 16–20 h. Cells were lysed by sonication, lysates were then cleared by centrifugation at 14,000 rpm for 1 h and the supernatants were incubated with glutathione sepharose (GE Healthcare) for purification, with recombinant proteins eluted in 10 mM reduced glutathione (MilliporeSigma). Protein concentrations were measured using Pierce Coomassie Plus assays. MLL1 complex and PRC2 complex were purchased (Active Motif).
Determination of IC50 for NSD2i
A MTase-Glo Methyltransferase Assay kit (Promega) was used to measure the IC50 for NSD2i with different lysine methyltransferases. A 10 μl reaction system was assembled with 25 nM lysine methyltransferase enzyme (or enzyme complex), 0.5 µM SAM, 100 nM mononucleosome (EpiCypher), MTase-Glo reagent (1×) and a series of diluted NSD2i in reaction buffer (containing 50 mM Tris pH 8.0, 20 mM KCl, 5 mM MgCl2 and 10% glycerol) in a white 384-well microplate (Corning). NSD2i was serially diluted from 30 μM to 0 μM in threefold concentrations. Each reaction was performed in triplicate and incubated for 3 h at 30 °C. Subsequently, 10 μl MTase-Glo Detection solution was added and incubated for 1 h at room temperature. The reactions were detected by luminescence, and the relative activity of enzymes calculated with the initial data. IC50 values were analysed using Prism (GraphPad software, v.10.5.0).
NSD2i treatment of organoids
We pretreated mouse and human organoids with NSD2i for 12 or 21 days, respectively, before 5 days of co-treatment with NSD2i and enzalutamide. Organoids were dissociated into single cells using TrypLE and seeded at a density of 1,000 cells per well (mouse) in neuroendocrine organoid culture medium without DHT in a 96-well low-attachment dish with 5% Matrigel (mouse) or as a total of 25,000 cells (human) in a 50 µl 80% Matrigel button in human neuroendocrine culture medium without DHT. DMSO or NSD2i at concentrations ranging from 0.3 µM to 10 µM were added on the day of plating and replenished every other day. Following pretreatment, organoids were dissociated into single cells and seeded at a density of 5,000 cells per well (mouse) or 10,000 cells per well (human) in a 96-well low-attachment dish in the same culture medium. The next day, DMSO or the same concentration of NSD2i were dispensed together with enzalutamide at threefold dilutions ranging from 3 nM to 10,000 nM in 0.1% DMSO. Cell viability was assayed after 5 days using CellTiter-Glo 3D (Promega, G9683), with luminescence measured by a GloMax Explorer multimode plate reader (Promega, v.3.1.0).
To detect apoptotic cell death, organoids were dissociated into single cells and seeded at a density of 2.5 × 104 cells per well (human) in a 96-well low-attachment dish in the same culture medium. DMSO (negative control), 10 µM bortezomib (Cayman Chemical, 10008822) as a positive control or NSD2i was dispensed together with a threefold dilution series of enzalutamide from 3 nM to 10,000 nM in 0.1% DMSO. Apoptotic cell death was assayed after 24–48 h treatment using a Caspase-Glo(R) 3/7 3D assay (Promega, G8981), with luminescence measured by a GloMax Explorer multimode plate reader (Promega, v.3.1.0). Luminescence values were first normalized to the DMSO-treated controls and then compared with 10 µM bortezomib treatment, which induces 100% apoptotic cell death after 24 or 48 h. The resulting signals normalized to a percentage of maximal response were plotted using Prism (GraphPad software, v.10.5.0).
To investigate epigenomic changes after drug treatment, non-neuroendocrine NPPO-1nonNE, NPPO-7, NPPO-8 and NPPO-9 organoids were seeded at density of 5,000 cells per well in 96-well ultralow attachment microplates in 5% Matrigel in organoid culture medium without DHT. On the day after seeding, DMSO, 1 µM enzalutamide or the combination of 1 µM enzalutamide and 1 µM NSD2i was added into the culture medium and replenished every other day. On day 40 after treatment, organoids were collected for fixation by 10% formalin for immunofluorescence staining or for flash-freezing with liquid nitrogen for western blot analysis. Western blotting for AR protein using the antibody listed in Supplementary Table 6 was performed using the methods described above.
DHT agonist treatment of organoids
To investigate whether NSD2 targeting restores AR activity, NPPO-1NE, NPPO-2 and MSKPCa10 organoids transfected with sgControl or sgNsd2 were cultured in organoid culture medium without DHT for three passages. The organoids were then dissociated and seeded at density of 5,000 cells per well in 96-well ultralow attachment microplates in 5% Matrigel in organoid culture medium without DHT. One day after seeding, 100 nM DHT or DMSO was added to the medium and replenished every other day. Cell numbers were counted every other day using a TC20 automated cell counter.
Multidrug synergy analysis
To analyse synergy between NSD2i and enzalutamide, the multidose combination response data generated from CellTiter-Glo 3D assays were input into SynergyFinder (v.3.0; https://synergyfinder.fimm.fi), a web application for interactive analysis and visualization of multidrug combination response data. NSD2i and enzalutamide drug combination responses were calculated based on the Bliss reference model using SynergyFinder64. Deviations between observed and expected responses with positive and negative values denote synergy and antagonism, respectively. For estimation of outlier measurements, the cNMF algorithm65 implemented in SynergyFinder was used.
Grafting assays
To generate tumours in vivo, mouse NPp53 or human prostate organoids were grafted into 6–8-week-old NOD/SCID male mice (NOD.CB17-PrkdcScid/J, Jackson Laboratory, 001303). NOD/SCID mice underwent surgical castration at 7 days before grafting. For mouse grafts, 1 × 106 dissociated organoid cells in 100 µl hepatocyte culture medium and 5% Matrigel were subcutaneously injected into the flank using a 1 ml syringe with a 25 G needle (BD, 305122). For human grafts, 3 × 106 dissociated cells in 100 µl 60% Matrigel and 40% hepatocyte culture medium were injected. Tumour sizes were measured with a digital caliper. Mice were randomly assigned to cohorts for treatment. In each cohort, 20–24 mice whose tumour volume had reached about 250 mm3 (mouse grafts) or 80 mm3 (human grafts) at week 2 after grafting received either 10 mg kg–1 enzalutamide (TargetMol, T6002) or 0.5% DMSO (MilliporeSigma, D2650) by daily gavage through a 20 G needle (Roboz, FN-7910) for 14 days (mouse grafts) or 56 days (human grafts). Enzalutamide or DMSO was suspended in 1% carboxymethylcellulose (MilliporeSigma, 419281) and 0.1% Tween 80 (MilliporeSigma, P4780) in distilled water. At the end of drug treatment, tumours were collected and imaged under a stereomicroscope (Olympus SZX16) with digital camera (Olympus, DP71), and image acquisition was performed with an Olympus DP Controller (v.3.3.1). Investigators were blinded to data collection and analysis. Tumour sizes never exceeded the maximal limit of 8,000 mm3 permitted by the IACUC at Columbia University Irving Medical Center. Tumour tissues were fixed in 10% formalin for 24–48 h and processed at the Columbia Molecular Pathology Core Facility. Tumour volumes were calculated using the formula:
$${{\rm{V}}{\rm{o}}{\rm{l}}{\rm{u}}{\rm{m}}{\rm{e}}={\rm{w}}{\rm{i}}{\rm{d}}{\rm{t}}{\rm{h}}}^{2}\times \frac{{\rm{l}}{\rm{e}}{\rm{n}}{\rm{g}}{\rm{t}}{\rm{h}}}{2}$$
Tumour growth curves were plotted using Prism (GraphPad software, v.10.5.0). Two-way ANOVA and Tukey’s multiple comparison post hoc testing were used to compare differences between means.
Drug treatment assays in vivo
For drug treatment, NSD2i, enzalutamide or DMSO were suspended in 1% carboxymethylcellulose and 0.1% Tween 80 in distilled water. To generate human prostate organoid grafts, 3 × 106 dissociated cells in 100 µl 60% Matrigel and 40% human hepatocyte culture medium were subcutaneously injected into the flank of castrated NOD/SCID male mice. To access NSD2i potency and specificity in vivo, 12 mice grafted with WCM1262 organoid cells with a tumour volume of about 100 mm3 at 3 weeks after grafting received 75 mg kg–1, 150 mg kg–1 or 300 mg kg–1 NSD2i or 0.5% DMSO by daily gavage through a 20 G needle for 5 days. Tumours were then collected and dissociated into single cells for western blot analyses of histone marks.
To investigate response to NSD2i and enzalutamide, we used cohorts of 24 mice for each organoid line examined. In each cohort, mice with a tumour volume of about 80–86 mm3 at 2 weeks after grafting received either 150 mg kg–1 NSD2i or 0.5% DMSO by daily gavage through a 20 G needle for 14 days. The 12 mice in the NSD2i treatment group were then subdivided into two groups for treatment with 150 mg kg–1 NSD2i alone or 150 mg kg–1 NSD2i with 10 mg kg–1 enzalutamide for another 4 weeks. The 12 mice from the DMSO control group were also subdivided for further treatment with DMSO or with 10 mg kg–1 enzalutamide for another 4 weeks. The tumours were collected and imaged using a stereomicroscope (Olympus SZX16) with a digital camera (Olympus DP71), using an Olympus DP Controller (Olympus, v.3.3.1.292). Tumour sizes never exceeded the maximal limit of 8,000 mm3 permitted by the IACUC at Columbia University Irving Medical Center. Tumour tissues were fixed in 10% formalin for 24–48 h and processed at the Columbia Molecular Pathology Core Facility. Tumour volumes and growth curves were calculated as described above. Two-way ANOVA and Tukey’s multiple comparison post hoc testing were used to compare means among groups.
Detection of proliferation and apoptosis in tumour sections
Tumour sections were stained for Ki67 or CC3 together with at least one lineage marker: CHGA, SYP or CD56. Antibodies used are listed in Supplementary Table 6. Tiled scans of whole tumour tissue sections were imaged using a Leica STELLARIS 5 confocal microscope (Leica Microsystems), and image acquisition was performed using Leica Application Suite X (LAS X v.4.5.0). For quantitation of proliferation and apoptosis, six regions of interest were chosen randomly on the whole slide image using QuPath (v.0.5.1)66. A machine-learning classifier was trained for identification of Ki67+ or CC3+ cells using the built-in functions in QuPath, and the percentage of Ki67+ or CC3+ cells were measured in each region of interest. An average percentage out of six regions was determined per xenograft specimen and plotted using Prism (GraphPad software, v.10.5.0).
Pre-processing of scRNA-seq and snRNA-seq data
scRNA-seq and snRNA-seq samples were processed independently using scanpy (v.1.9.1) for Python (v.3.9)67. Cells with >1,000 detected genes and 1,000 unique molecular identificer (UMI) counts were retained, whereas cells with >10,000 detected genes or 50,000 UMI counts and with >20% of mitochondrial gene content were discarded. For doublet detection and removal, we used the Scrublet (v.0.2.2) algorithm as implemented in scanpy, applied to each sample independently. Each sample was then subsampled by retaining 3,000 cells that passed quality control, using the ‘subsample’ function with random state set to 666 as implemented in scanpy. All samples processed with the same technology (that is, single-cell or single-nuclei) were merged and UMI counts per cell were converted to sum to 1e4 and log-normalized.
Reverse engineering of prostate organoid regulatory networks
For each scRNA-seq sample, a shared neighbours graph was built with knn = 15 to select cells with the most similar transcriptional profiles and to merge them to generate high-resolution ensembles of cells (metacells). This approach augments the number of detected genes per cell, which usually is very low owing to technological dropout bias (<20%), thus increasing the number of targets that can be recovered by reverse engineering of regulatory networks. Metacell profiles were computed on normalized data, merged into UMI counts, and transformed to CPM for downstream analysis.
A sample-specific regulatory network (interactome) was reverse engineered from the resulting metacell CPM profiles (n = 500) using ARACNe-AP19, the most recent implementation of the ARACNe algorithm18, with 200 bootstraps, a mutual Information (MI) P value threshold P ≤ 10–8 and data processing inequality (DPI) enabled. Regulatory proteins (RPs) were selected into manually curated protein sets, including transcription factors (TFs), co-TFs or chromatin-remodelling enzymes, using the Gene Ontology (GO) identifiers GO:0003700 and GO:0003712. Each RP regulon (RP gene targets) was integrated across all the reverse-engineered networks to realize one final network. To avoid bias due to different regulon sizes, regulons were pruned to include only the 50 highest likelihood targets17, and regulons with <50 targets were excluded from the analysis. Mouse prostate cancer scRNA-seq and snRNA-seq gene expression profiles were scaled independently and transformed to protein activity profiles using the metacell-derived regulatory network and the VIPER algorithm as implemented in pyVIPER (viper-in-python, v.1.0.9)68. All samples were merged to be projected on a two-dimensional plane using Diffusion Maps69. Diffusion Maps were computed using a KNN graph constructed from Harmony-corrected principal components, with single-cell technology (that is, single-cell or single-nucleus) specified as the batch variable70.
To recover cell identities, clusters of cells that share the same regulatory programs were identified using automated community detection of cell populations (ACDC)71, a scanpy-compatible tool that identifies optimal clustering parameters based on heuristics using acdc_py (v.1.1.0; https://github.com/VasciaveoLab/acdc_py). Specifically, ACDC performed a grid search analysis to tune the Leiden clustering algorithm resolution parameter to maximize the average of within-cluster silhouette scores across each candidate optimal clustering solution. A high silhouette score is an indication that clustered cells have homogenous profiles, as sampled from the same cell population. The optimal solution produced three major clusters.
Single-sample gene set enrichment analysis and AR targets analysis
To perform enrichment for AR canonical targets57, pseudo-bulk samples were created by averaging the expression of each gene in each individual sample from snRNA-seq data, processed by gene set enrichment analysis (GSEA) with normalized enrichment scores and nominal P values determined by 1,000 permutations of gene labels using permutation tests.
Integration of CUT&Tag and VIPER analysis of snRNA-seq data
Histone mark count matrices were processed using limma ‘voom’ (v.3.54.2). Linear models were fitted for each gene based on the voom-transformed data with moderated t-statistics computed using the eBayes method from limma. A contrast matrix was built to compare differential H3K36me2 histone marks between untreated cells and NSD2 knockout or H3.3K36M transfection. Top-ranked genes were extracted using the topTable function and genes with log[fold change] < −0.5 and false discovery rate < 0.1 were retained. This gene set was then used for GSEA analysis on gene expression signatures computed as differential between cluster 1 and cluster 3 across conditions (for example, NPPO-1NE sgNSD2 cluster 1 versus NPPO-1NE sgCtrl cluster 3; Extended Data Fig. 6). Normalized enrichment scores and nominal P values were determined by 1,000 random permutations of gene labels using permutation tests. For differential gene expression analysis Seurat (v.4.1.3) was used, with parameter test.use set to DESeq2 (v.1.28.0) in the ‘FindMarkers’ function.
Statistics and reproducibility
All experiments described in this study include sufficient biological replicates to draw statistically meaningful conclusions. Detailed statistical methods, including statistical tests used, error bar definitions, exact sample size (n values), P values and whether the test is one-sided or two-sided, are described in the Methods and corresponding figure legends. Statistical methods were not used to predetermine sample size. Source data for all western blot and gel images are provided in Supplementary Fig. 1. All western blots, H&E and immunofluorescence staining were performed at least three times on different batches of samples to ensure that the results are reproducible. Representative images shown in the figures correspond to reproducible general conclusions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.