Mice
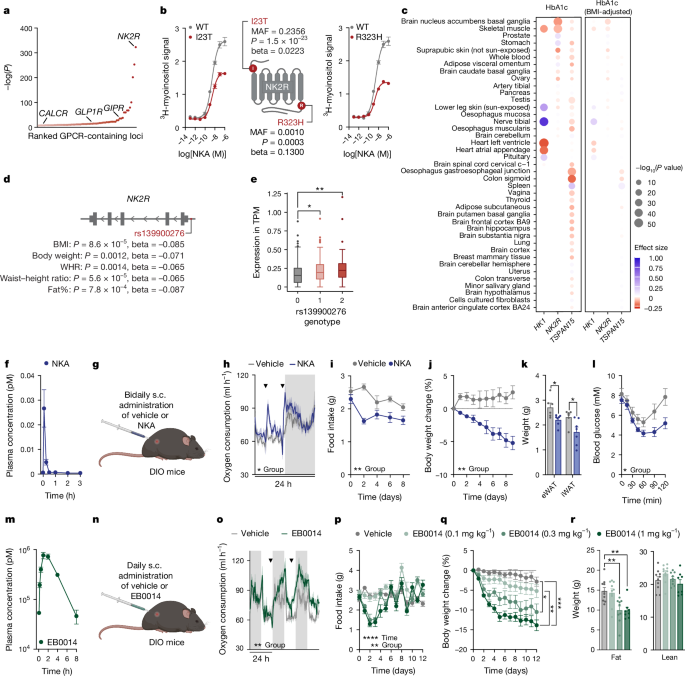
Mouse studies performed at the University of Copenhagen were approved by The Danish Animal Experiments Inspectorate (permit number: 2018-15-0201-01441 and 2023-15-0201-01386) and the University of Copenhagen. Animal experiments performed at the University of Michigan were approved by the University of Michigan Committee on the Use and Care of Animals (protocol no. 00011066) and in accordance with Association for the Assessment and Approval of Laboratory Animal Care and National Institutes of Health guidelines. Studies in Fig. 1pâr using EB0014 were conducted by Gubra. Mice were housed in solid bottom cages with environmental enrichment at 22â°C (±2â°C) in 55% (±10%) humidity and a 12-h light:dark cycle (light from 06:00 to 18:00). Mice had ad libitum access to water and standard chow diet (SAFE D30, Safe Diets) or 60% HFD (Rodent Diet with 60âkcal% fat, D12492i, Research Diets) to induce obesity as indicated in the figure captions and results. For studies with DIO mice, mice were challenged with HFD for a minimum of 12 weeks starting from 8 weeks of age and were used in pharmacology studies once the mean cohort size was at least 35âg. Groups were randomized for vehicle and compound treatment. Pharmacological DIO studies in wild-type male and female mice were performed on animals with a C57Bl/6âNRj genetic background (Janvier Labs). The description of backgrounds for the genetic models used are as follows: B6.V-Lepob/JRj (ob/ob) mice and RjOrl:SWISS (CD-1) mice were purchased from Janvier Labs. B6.129S4-Mc4rtm1Lowl/J (Mc4r-knockout), Ccktm1.1(cre)Zjh/J, B6.129-Leprtm3(cre)Mgmj/J, Calcrtm1.1(cre)Mgmj/J, B6;129-Gt(ROSA)26Sortm5(CAG-Sun1/sfGFP)Nat/J and Gt(ROSA)26Sortm1(CAG-EGFP/Rpl10)Dolsn mice were purchased from the Jackson Laboratory. The Ucp1-knockout line was provided by K. Kristiansen. B6N-Tacr2tm1Zpg (Nk2r-floxed) mice were generated by GenOway on a C57Bl/6N background by inserting loxP sites around exon 1, which encodes 130 out of the 384 amino acids in the full-length receptor. Ucp1-knockout, Nk2r-knockout and Nk2r-floxed lines were bred in-house at 22â°C (â±â2â°C). Ccktm1.1(cre)Zjh/J and B6.129-Leprtm3(cre)Mgmj/J mice were crossed with Gt(ROSA)26Sortm1(CAG-EGFP/Rpl10)Dolsn mice to create CckCreL10 GFP and LeprCre L10 GFP mice respectively. Calcrtm1.1(cre)Mgmj/J mice were crossed with B6;129-Gt(ROSA)26Sortm5(CAG-Sun1/sfGFP)Nat/J to create CalcrCre Sun1 GFP mice. Studies were generally performed at 22â°C (±2â°C). Studies in Ucp1-knockout mice and the ob/ob one time injection and subcutaneous/ICV crossover study were performed in thermoneutrally (29â°C (±2â°C)) housed mice.
Mice were trained with mock injections for all injection studies for at least 5 days. NKA (1âmgâkgâ1; Almac) dissolved in Gelofusine (B. Braun) was administered subcutaneously twice daily for 9 consecutive days. EB0014 (synthesized by Almac) was dissolved in DMSO to a 17âmgâmlâ1 solution and further in 0.9% saline with 3% BSA. EB0014 was subcutaneously administered once daily at 1âmgâkgâ1 for indirect calorimetry measurements and at 0.01âmgâkgâ1, 0.1âmgâkgâ1, 0.3âmgâkgâ1 and 1âmgâkgâ1 for the dose-dependent weight loss study.
EB1002 (synthesized by Novo Nordisk or PolyPeptide) was dissolved in 8âmM phosphate and 240âmM propylene glycol (pH 8.2) and administered once, daily for 7 or 21 consecutive days or every other day for 21 days via subcutaneous injections at 40ânmolâkgâ1 or 325ânmolâkgâ1 between 15:00 and 16:00. The NK2R antagonist, saredutant (SR 48968, MedChemExpress) was injected into the peritoneum at 5âmgâkgâ1 in 0.9% saline with 1% Tween80 30âmin prior to EB1002 injections. Body weight, blood glucose and food weight were taken immediately before injections and 24âh after injections. The amount of food for pair-fed groups was determined on the basis of at least two previous studies. To mimic a diet intervention, DIO mice were switched to chow diet 5 days prior to first injection and compared to DIO mice maintained on HFD. The effect of EB1002 on food intake in Extended Data Fig. 5a was assessed in overnight-fasted mice that were refed at the time of injection.
The insulin tolerance test was performed in DIO mice after the 9-day injection regimen, 12âh after the final injection with 1âmgâkgâ1 NKA. Insulin tolerance test was carried out on 4-h fasted mice by intraperitoneal injection of 0.75âUâkgâ1 insulin. Twenty-four hours prior to the glucose tolerance test, DIO mice were subcutaneously injected with vehicle or 325ânmolâkgâ1 EB1002. Vehicle-treated mice had either ad libitum access to HFD or were pair-fed to EB1002-treated mice. Glucose tolerance test was performed the following day on 4-h fasted mice by intraperitoneal injection of 1âgâkgâ1 glucose. Insulin levels were measured using the Mouse/Rat Insulin Kit (mesoscale) according to the manufacturerâs protocol.
GLP-1, leptin, glucagon and PYY were measured in plasma samples using the U-PLEX Gut Hormone Combo 1 (ms) SECTOR Kit (MSD, K15307K-1) according to the manufacturerâs protocol.
To evaluate the aversive potential in mice, a saccharin-conditioned taste avoidance was performed as described63. On the conditioning day, mice were exposed to a new flavour (0.15% saccharin) followed by a subcutaneous injection of vehicle, 10ânmolâkgâ1 semaglutide or 325ânmolâkgâ1 EB1002.
Liquid phase gastric emptying was performed in 4-h fasted mice. Mice received an injection of vehicle or EB1002 30âmin prior to an oral gavage of 4âmg paracetamol in 0.9% NaCl. Blood samples were collected via the tail vein 15âmin after gavage and paracetamol levels were measured using Acetaminophen L3K assay kit (Sekisui Diagnostics, 506-30) according to the manufacturerâs protocol.
Tissue-specific insulin signalling was assessed in mice 18âh after injection with vehicle or EB1002. 2âh fasted mice were sedated with 75âmgâkgâ1 pentobarbital (intraperitoneal injection) and received a retro orbital injection with 1.5âUâkgâ1 insulin. Tissues were dissected 10âmin after insulin administration, snap-frozen and analysed as described in âImmunoblottingâ.
Total lean and fat mass were measured by magnetic resonance imaging using the minispec LF90 II body composition analyser (Bruker).
Metabolic characterization
Oxygen consumption, carbon dioxide production and food intake were recorded using the Promethion core system (Sable Systems International) with data processing using OneClickMacroV2.52.1 and Macrointerpretersetup_v2_47 or the Phenomaster Home Cage System (TSE Systems) with PhenoMaster software v.8.2.9. Generally, mice were transferred to training cages 3â7 days prior to the measurement and were allowed to acclimate in the measurement chambers for at least 24âh or until a stable baseline was observed. Fatty acid oxidation was calculated using the formula64: energy expenditure (kcal/h)âÃâ(1-RER)/0.3.
Core body temperature and gross motor activity measurements
Continuous body temperature and motor activity were measured with G2 E-Mitter telemetric devices (Starr Life Sciences) or with radio frequency identification (RFID) temperature transponders (UCT-2112 microchips, Unified Information Devices). Probes were implanted into the peritoneal cavity under sterile conditions. In brief, mice were anaesthetized with isoflurane and received 5âmgâkgâ1 Rimadyl (ScanVet, 027693) and 8âmgâkgâ1 Lidocaine (AstraZeneca). The sterile probe was inserted into the peritoneum via a midline incision. After closure, mice were allowed to recover on a heated surface and received 5âmgâkgâ1 Rimadyl for 3 consecutive days. Mice were allowed to recover for at least 7 days before study start. E-mitter data was recorded by placing ER4000 Receivers under the cages within the TSE cabinet. Temperature data was integrated in the Phenomaster software. RFID temperature transponder data was recorded by using UID Mouse Matrix system (Unified Information Devices) in combination with the Sable system.
Triple-chip study
RFID temperature transponders (UCT-2112 microchips, Unified Information Devices) were surgically implanted and anchored in female mice for continuous and simultaneous measurement of temperature in three distinct anatomical locations. Surgical and post-operative procedures were performed as described above. Ventromedial abdominal chip (abdominal temperature): following a midline incision, the chip was inserted in the abdominal cavity. Distolateral femoral chip (hindlimb temperature): following a transverse left gluteal incision, a subcutaneous lateral femoral pocket was prepared, and the chip was inserted. Dorsomedial intrascapular chip (interscapular temperature) and bilateral BAT denervation: following a midline dorsal incision, the interscapular BAT was detached from the underlying muscle layer. Bilateral denervation was performed as described65. The chip was inserted with the tip containing the temperature probe placed between the BAT and the dorsal muscle. The three temperatures were recorded using the UID Mouse Matrix system (Unified Information Devices).
Brain cannulation and ICV injections
For administration of compounds directly into the brain of awake mice, a cannula was placed into the cerebral ventricle. B6.V-Lepob/JRj (ob/ob) mice were anaesthetized with isoflurane and received 5âmgâkgâ1 Rimadyl (ScanVet, 027693) and 8âmgâkgâ1 Lidocaine (AstraZeneca). Mice were fixated in a stereotaxic frame (Kopf Instruments) and the scalp was opened to expose the skull. Coordinates were zeroed on bregma and moved to â0.3âmm in the anteroposterior axis and â1.0âmm in the mediolateral axis. A small hole was drilled into the skull and the guide cannula (C315GS-4/Spc 2âmm, PlasticsOne) was inserted and fixated using a G-bond layer (GC America) and G-ænial universal flow (GC America). Finally, a dummy (C315DCS-4/Spc 2.5âmm, PlasticsOne) was screwed onto the guide cannula and mice were allowed to recover on a heated surface and received 5âmgâkgâ1 Rimadyl for 2 consecutive days following the surgery. To test the correct placement of the cannula, mice received an infusion of 1.5âμl of 24âμM angiotensin II (Sigma) in artificial cerebrospinal fluid (CSF) (Harvard Apparatus) via an injector (C315IS-4/Spc 2.5âmm, PlasticsOne) and an infusion pump (Harvard Apparatus) at a rate of 2âμlâminâ1 5 days after surgery. After the infusion, the injector was left in place for an additional 45âs to minimize backflow. Then, the injector was removed, and the dummy was placed on the cannula. Mice were monitored for angiotensin II-induced water intake and responsive mice were subsequently used for ICV injection studies. One week prior to study start, mice were housed at thermoneutrality. Mice first received a single subcutaneous injection of vehicle or 325ânmolâkgâ1 EB1002 and after a wash-out period they were infused with 1.5âμl artificial CSF or 2ânmol EB1002 in 1.5âμl artificial CSF as described above in a crossover design.
AAV injections into the DVC of Nkr2-floxed mice
Nk2rfl/fl mice were anaesthetized using isoflurane. Mouse heads were oriented at a 90° angle and an incision was made at the caudal aspect of the skull to expose the brainstem. Using the obex of the skull as a guide, 50ânl of AAV-GFP or AAV-CMV-CRE-GFP were injected into each site of the DVC at a depth (z) of 0.350âmm. Virus was injected at a rate of 15â35ânlâminâ1. The pipette remained in the DVC for an additional 3 min to allow viral particles to disperse, and then the pipette was slowly removed. Mice received prophylactic analgesic carprofen (5âmgâkgâ1) before and for 24âh after surgery and were monitored for 10 days following the procedure to ensure recovery for surgical intervention. Mice were fed a 60% HFD starting 6 weeks after surgery for 5 weeks. To assess the acute food intake response to EB1002, mice were injected subcutaneously with either vehicle or 325ânmolâkgâ1 EB1002 in a crossover design. Food intake was measured at hours 0, 1, 2, 4 and 24 and body weights were recorded at 0 and 24âh post-injection. After termination, hit sites of all mice were confirmed using immunohistochemistry and mice with a confirmed bilateral hit site were included in the analysis.
Hyperinsulinaemicâeuglycaemic clamp and peripheral glucose uptake
Hyperinsulinaemicâeuglycaemic clamps were performed in conscious, unrestrained male mice at 24 weeks of age as previously described66. In short, catheters were implanted under aseptic conditions into the right jugular vein (C20PU-MJV1458; Instech) and the left common carotid artery (C10PU-MCA1459; Instech), exteriorized in the scapular region and secured using a dual-channel vascular access button (VABM2B/25R25; Instech) under general isoflurane anaesthesia. Mice were subcutaneously injected preoperatively with Carprofen (5âmgâkgâ1, Norodyl Vet, Scanvet) for analgesia and a mixture of Lidocaine (7âmgâkgâ1, Xylocain, AstraZeneca) and Bupivacaine (7âmgâkgâ1, Marcaine, Orifarm) at the incision sites for local anaesthesia. Mice were allowed to recover for 7â10 days. Mice were treated with either vehicle or 325ânmolâkgâ1 EB1002 24âh prior to clamp experiment. Clamp studies were performed in 4âh fasted mice. At 15âmin and 5âmin before the start of the clamp, blood samples were taken for determination of basal glucose and insulin levels. At 0âmin, the clamp was initiated with continuous infusion of human insulin (4mUâkgâ1âminâ1, Actrapid; Novo Nordisk, Denmark). Donor red blood cells were washed and used to compensate for the blood loss to experimental mice during the repeated sampling (5âμlâminâ1 of 50% RBC in 10âUâmlâ1 heparinized saline). Blood glucose samples were taken every 10âmin (Contour XT, Bayer) and blood glucose was then adjusted using a variable infusion of 50% glucose. Both human and mouse insulin levels (Mercodia) were determined at 100 and 120âmin. After 120âmin, a 13âμCi bolus of 2-[1-14C]-deoxy-d-glucose was given and blood samples taken at 122, 125, 135, 145 and 155âmin and specific activity for 2-[1-14C]-deoxy-d-glucose was determined in these samples. After euthanization, tissues for tissue-specific 2-[1-14C]-deoxy-d-glucose uptake were sampled and snap-frozen in liquid nitrogen. Samples from tissue-specific glucose uptake were processed as previously described66.
Pharmacological pre-toxicology evaluation
For the evaluation of potential toxicological effects CD-1 outbred mice were daily either injected with vehicle or with increasing concentrations of EB1002 (150ânmolâkgâ1 (2 consecutive days), 300ânmolâkgâ1 (2 consecutive days), 600ânmolâkgâ1 (2 consecutive days), 1,200ânmolâkgâ1 (2 consecutive days), 2,400ânmolâkgâ1 (2 consecutive days), 4,800ânmolâkgâ1 (2 consecutive days) and finally 7,500ânmolâkgâ1 (3 consecutive days)). Liver, spleen, heart, kidney, oesophagus, stomach, duodenum, colon, testis, cornea, bladder and thymus were dissected, fixed in 10% formalin and stained with haematoxylin and eosin for pathohistological analysis performed by the Histology department, HistoCore, at the University of Copenhagen. Slides were compared pairwise. Serum liver enzymes were analysed by the Veterinary Diagnostic Laboratory core at the University of Copenhagen.
Quantification of faecal cholesterol and triacylglycerides
Faecal samples were homogenized in cold methanol containing butylated hydroxytoluene (1âmgâmlâ1). After centrifugation (10âmin at 10,000g, 4â°C), the supernatants were transferred into fresh vials and the methanol was evaporated by vacuum centrifugation. Pellets were re-dissolved in 0.1âM potassium phosphate, pH 7.4, 0.05âM NaCl, 5âmM cholic acid and 0.1% Triton X-100. Cholesterol and triacylglycerides were quantified using colorimetric kits from DiaSys (Cholesterol (113009910704) and triacylglycerides (157109910026)) according to the manufacturerâs instructions.
NKA and compound detection in the blood
Pharmacokinetic profiles were determined in lean mice. Mice were subcutaneously dosed with 1âmgâkgâ1 NKA, 666.3ânmolâkgâ1 EB0014, 325ânmolâkgâ1 EB1001 and 325ânmolâkgâ1 EB1002. Blood samples were taken starting at 5âmin until 60âh as indicated in respective graphs. Plasma levels of NKA were determined using a Human Neurokinin A kit Elisa Kit (Ray Biotech). Plasma levels of EB0014, EB1001 and EB1002 were determined using LC-MS after precipitation with acetonitrile containing 0.1% formic acid.
In situ hybridization
Wild-type mice were anaesthetized with 75âmgâkgâ1 pentobarbital and transcardially perfused with 4% paraformaldehyde (PFA) in PBS. Whole brains were dissected, further fixated in 4% PFA in PBS for 48âh at 4â°C and subsequently dehydrated using the automated Excelsior AS tissue processor (Thermo Scientific) and embedded in paraffin. Paraffin-embedded brains were cut into 4 μm sections using the Microm Ergostar HM 200 (Marshall Scientific) and mounted onto glass slides. After deparaffination and rehydration, RNA molecules were detected using the RNAscope Multiplex Fluorescent V2 Assay kit (ACDbio) according to manufacturerâs protocol. Nk2r, Calcr and Glp1r were detected with the RNAscope probes Mm-Tacr2 (441311), Mm-Calcr-C2 (494071-C2) and Mm-Glp1r-C3 (418851-C3), and 3-plex Negative probe (320871) and 3-plex-mouse Positive probe (320881, all ACDbio) were used as negative and positive control respectively. Signals were visualized with fluorophores at 570ânm (OP-001003) for channel 1, 520ânm (OP-001006) for channel 2 and 690 for channel 3 (OP-001001, all 1:1000, Akoya Bioscience). Sections were mounted with ProLong Gold antifade reagent with DAPI (P36935, Invitrogen). For analysis, 4 mice were used and 2â3 sections per animal were imaged.
Immunohistochemistry
Mice were fasted overnight and injected subcutaneously with vehicle or 325ânmolâkgâ1 2âh prior to euthanasia. Mice anaesthetized with isoflurane and transcardially perfused with PBS followed by 4% PFA in PBS. Whole brains were dissected, further fixated in 4% PFA in PBS for 4âh at 22â°C. After 48âh in a 30% sucrose solution, brains were cut into 30âµm sections using a sliding microtome SM2010R (Leica) with a freezing stage and temperature controller (BFS40-MPA, Physiotemp). Free floating sections were blocked in 3% donkey serum with 0.1% Triton X-100 in PBS and incubated overnight at room temperature using the following antibodies, against FOS (Cell Signalling, 2250, 1:1,000) and GFP (Aves Laboratories, 1020, 1:1,000). Sections were washed, incubated with a secondary antibody conjugated to Alexa Fluor 488 and 568 (Invitrogen, A-11039, A-11011, 1:250) and mounted.
To assess neuronal degradation in the DVC of Nk2rDVC-GFP and Nk2rDVC-cre mice, the Fluoro-Jade C (FJC), RTD Ready-to-Dilute Staining Kit for identifying Degenerating Neurons (VWR) was applied according to manufacturerâs instructions. Fluoro-Jade C positive neurons were counted for quantification of neuronal degradation.
Microscopy
Fluorescence microscopy was performed using a Zeiss Axio Observer microscope with Axiocam 702 mono camera or with an Olympus BX53 microscope.
Whole-brain FOS imaging
Two-hour fasted obese wild-type mice received a single subcutaneous dose of vehicle or 325ânmolâkgâ1 EB1002 before being terminated and perfused with 4% PFA 2âh later. The brains were subsequently isolated, postfixed, and transferred to Gubra. At Gubra, the samples were cleared, stained for FOS, and imaged at single-cell resolution using a light sheet microscope as previously described40. The data from individual brains was mapped into an average mouse brain atlas template, and the number of FOS labelled cells was quantified in more than 800 brain regions. For the calculation of fold changes, brain regions without FOS signal (nâ=â25) were excluded.
Immunoblotting
Protein was isolated and western blots were run as described previously67. Proteins were detected using the following antibodies, AKT (Cell Signaling, 9272, 1:1,000), pAKT T308 (Cell Signalling, 9275, 1:1,000), Tyrosine hydroxylase (Abcam, ab137869, 1:1,000), UCP1 (Abcam, ab10983, 1:7,500) and peroxidase-conjugated AffiniPure Goat Anti-Rabbit IgG (H+L) (Jackson Immuno Research, 111-035-144, 1:5,000). Images were acquired using an Odyssey Fc Imager (LI-COR). Uncropped images can be found in the source data.
snRNA-seq
Mice were injected subcutaneously with vehicle or 325ânmolâkgâ1 EB1002 2âh prior to euthanasia. After decapitation, the brain was removed, and the DVCs were isolated from the brainstem under a surgical microscope. A 1âmm coronal section of the hindbrain from bregma â7.0 to â8.0âmm was cut with a razor blade. From the coronal section, a 1.4âmm square containing the DVC was snap-frozen. Samples from the same experimental condition were pooled (nââ=ââ5 mice per sample). Nuclei were extracted as previously described68. Sorting of 2n nuclei was performed by flow cytometry using a BD FACSAria IIIu Influx cell sorter (BD Biosciences). The gating was set according to size and granularity using FSC and SSC to capture singlets and remove debris. To detect DraQ5-positive nuclei fluorescence was set at 647ânm and 670ânm. Each sample was sorted into separate tubes, each with a total of 20,000 nuclei per 40âµl. Sequencing libraries were generated using 10x Genomics Chromium Single-Cell 3â² Reagent kit according to the standardized protocol. Paired-end sequencing was performed using an Illumina NovaSeq 6000.
snRNA-seq analysis
Raw sequencing data were demultiplexed, aligned to the mouse reference genome GRCm38 (mm10) and counted using cellranger (version 7.0.0; 10x Genomics). Ambient RNA molecules were removed from cellranger raw count matrix files with cellbender:remove-background (v0.3)69. Filtered count matrices were analysed in R with Seurat (v4.3.0)70. Nuclei with at least 1000 detectable genes were retained. Genes expressed in at least 10 nuclei were retained. ScDblFinder (v1.15.4)71 with standard parameters was run on individual 10x lanes to remove doublets. The count data were normalized with NormalizeData and scaled with ScaleData function. Genes were then defined as variable using FindVariableFeatures and used as input into principal components analysis with RunPCA. The top 30 principal components were retained and used for further dimensionality reduction using RunUMAP and clustered using a resolution of 0.8 with FindClusters. To perform cell-type-specific quality control, the nuclei were split into two broad categories, neuronal and nonneuronal, using CellAnnotatorR(v), on the basis of the expression of neuronal marker gene Rbfox3 or Snap25 and the absence of nonneuronal markers. Neuronal data was further filtered by removing nuclei with unique molecular identifiers (UMIs) in the first and 99th percentile. Library complexity was calculated by dividing the log of the total genes detected per nuclei by the log of the total UMIs per nuclei. Nuclei in the first percentile of this metric were removed. Add_Mito_Ribo_Seurat was used to identify and remove nuclei with >1% mitochondria and ribosomal genes. We finally removed outliers by isOutlier with nmadsâ=â5 run on individual 10x lanes from the package SingleCellExperiment72. After all quality control, a total of 23,664 neurons remained. Neuronal cell types were labelled by projecting labels from a previously published dataset39 (GSE166649) using Seurat FindtransferAnchors and TransferData function.
Immediate early gene analysis
To calculate an IEG score, rapid primary response genes73 (Fosb, Npas4, Fos, Junb, Nr4a1, Arc, Egr2, Egr1, Maff, Ier2, Klf4, Dusp1, Gadd45g, Dusp5, Btg2, Ppp1r15a and Amigo3) were used to score each nuclei with the AddModuleScore function from Seurat. For each cluster-sample combination we calculated the average activity score which we then modelled by the interaction of cell type and treatment. Estimated marginal means were compared between treatments within each cluster. P values were corrected for multiple testing using the BenjaminiâHochberg method (adjusting for the number of cell populations).
Perturbed cell-type analysis
We used the scDist package to calculate the transcriptional distance between treatment and controls while also controlling for individual-to-individual variability74.
Primary cell studies
Primary white adipocytes were isolated for wild-type mice and cultures as described75. For in vitro oxygen consumption measurements, cells were replated in Seahorse XF96 Cell Culture Microplates (Agilent Technologies) on day 3 and oxygen consumption in response to vehicle or EB1002 (10ânM, 1âµM and 10âµM) was measured using a Seahorse XFe96 Extracellular Flux Analyzer (Agilent Technologies) on day 7 as described75. For in vitro glucose uptake, differentiated cells were starved for 2âh before treatment with Krebs-Ringer buffer containing 5âmM glucose, and vehicle or EB1002 (10ânM, 1âµM and 10âµM). After 2âh, cell media was incubated with a reaction mix mix (200âmM Tris-HCl, 500âmM MgCl2, 5.2âmM ATP, 2.8âmM NADP, and 6âμgâmlâ1 hexokinase and glucose-6-phosphate dehydrogenase mixture (10737275001, Roche Diagnostics)) for 15âmin and glucose content was measured spectrophotometrically at 340ânm (Hidex sense, Hidex). Glucose uptake was calculated on the basis of disappearance of glucose from the media. Cells have been tested negative for mycoplasma contamination.
Ex vivo lipolysis
To assess the effects of EB1002 on the lipolytic function of adipocytes, mature adipocytes were isolated from iWAT of wild-type chow-fed mice. In brief, adipose depots were minced and digested in Krebs-Ringer buffer containing 2% BSA and 0.2% collagenase type I (Worthington Biochemical) at 37â°C. Cells were passed through a 100-µm cell strainer, centrifuged (10âmin, 10g, 4â°C) and the floating mature adipocyte fraction was washed three times. Cells were incubated with vehicle, EB1002 (10ânM, 1âµM and 10âµM) or 50ânM isoproterenol for 2âh at 37â°C. Finally, non-esterified free fatty acids were measured in the medium using the Fujifilm NEFA HR R2 kit according to manufacturerâs protocol.
Macaque studies
The macaque studies were conducted in compliance with all federal regulations, including the US Animal Welfare Act. Studies were reviewed and approved by the OHSU/ONPRC Institutional Animal Care and Use Committee. The ONPRC is accredited by AAALAC International. For these studies, 10 rhesus macaques (5 males and 5 ovariectomized females,12â23 years of age with a body weight ranging from 7â24âkg) were pair-housed (1 male and 1 female) in custom designed cages (Carter2 Systems) in shared rooms under fixed photoperiodic conditions (lights on from 07:00 to 19:00). The cages meet the minimum European Union accepted standards for housing nonhuman primates (2.0âm2 enclosure size, 3.6âm3 enclosure volume, and 1.8âm enclosure height). Shelves, verandas, solid flooring and changeable plastic toys were available for the monkeys. The commercially available HFD (5L0P, Lab/Test Diets) was provided ad libitum twice every day. Food intake was measured daily, and animals were separated for 1â2âh periods when individual food intake was measured. Paired food intake was measured for the remaining feeding times when animals were socially pair-housed.
EB1001 was dissolved in 8âmM phosphate and 240âmM propylene glycol (pH 8.2) and administered for 8 consecutive weeks via subcutaneous injections between 08:00 and 09:00 prior to the morning meal. All animals were started on the 30ânmolâkgâ1 dose with every other day dosing (q48h) for 1 week, followed by daily dosing of the subsequent higher doses. Dose escalations were as follows (nmolâkgâ1): 60 (1 week), 90 (1 week), 120 (1 week), 240 (2 weeks), 480 (4 days) and 240 (10 days). Body weight was measured on a weekly basis using the same, calibrated digital scale. Heart rate and oxygen saturation were recorded in sedated animals using a pulse oximeter (Model 7500, Nonin Medical).
Blood samples were collected in conscious animals prior to morning meal (overnight fast) and daily dose administration. Blood glucose was measured on a Biosen clinical analyser (EKF Diagnostics) and C-peptide and insulin were measured on a Cobas e411 analyser (Roche Diagnostics). Remaining chemistry parameters alanine aminotransferase (ALT), aspartate transaminase (AST), total cholesterol (Chol), creatinine (CREA), glucose triglyceride (TG), blood urea nitrogen (BUN) and LDL cholesterol were analysed using the Pentra C400 (Horiba Medical).
Behaviour analysis was performed by members of the ONPRC Behavioural Sciences Unit blinded to the experimental design. Observations were taken directly on a mobile device and average behaviour scores were calculated on the basis of events such as anxiety, stereotypy, eye poking or withdrawn behaviour. Additional cage side observations included signs of nausea (gaping and hunched posture), emesis and stool consistency.
Association of missense variants with cardiometabolic traits
To assess whether the 4 missense NK2R variants (R3232H, I23T, V54I and A161T) are associated with cardiometabolic traits, their associations in T2D Knowledge Portal (HbA1c phenotype page, accessed 21 May 2024; https://t2d.hugeamp.org/phenotype.html?phenotype=HBA1C; RRID:SCR_003743)24 were queried. For each variant, only the associations that presented a P valueâ<â0.05 for the latest and largest European genome-wide association study (GWAS) per trait were included. Additionally, the minor allele frequency in five ancestries are reported: AFR, AMR, EAS, EUR and SAS retrieved from 1000 Genomes reference panel data from dbSNP30,76.
SNP-level associations in the HK1âNK2RâTSPAN15 region
Utilizing the latest and largest European GWAS of HbA1c available in T2D Knowledge Portal (query on 10/05/2024) (HbA1c phenotype page, accessed 21 May 2024; https://t2d.hugeamp.org/phenotype.html?phenotype=HBA1C (RRID:SCR_003743))24, summary statistics from Jurgens et al.29 were retrieved and 1,944 common variants (minor allele frequency > 1%) located 100 kilobases (kb) upstream from the HK1 transcription start site (TSS) and 100âkb downstream from the TSPAN15 TSS (10:70929740â71367422) were investigated further.
The number of lead, independent, genome-wide significant variants were assessed by performing LD clumping with with plink1.9 (ref. 77) and utilizing the European 1000 Genomes reference panel phase 3 version 5 (ref. 30). An r2 threshold of 0.01 and distance threshold of 1,000 kilobases (kb) were used.
Fine mapping of HbA1c associations in 10:70929740â71367422 locus with CARMA31, a software designed to correct for differences in LD between summary statistics and LD reference panels were performed. CARMA with the default settings, utilizing European 1000 Genomes reference panel phase 3 version 5 with annotations30 were performed, and those variants that presented a posterior inclusion probability ofâ>0.1 were investigated further. In dbSNP76, minor allele frequencies in five genetic ancestries were retrieved: AFR, AMR, EAS, EUR and SAS.
Finally, Open Target Genetics (OTG)78 was utilized to query the variant-to-gene (V2G) scores used for gene prioritization and the associations of causal variants with eQTLs.
Linkage disequilibrium analysis
All analyses were performed in Rstudio (2022.07.2â+â576) with R (4.1.3). Data were loaded and manipulated using data.table (1.14.2) and tidyverse (1.3.1). LD operations were performed using plink1.9 (ref. 77), ggLD (https://github.com/mmkim1210/ggLD), and LDLink79. All results can be reproduced by following the code available at https://github.com/MarioGuCBMR/nk2r_hk1_genetics.
TWAS of NK2R and HbA1c
Elastic Net model of SPrediXcan methods were used to predict the association between gene and traits80,81,82. The Elastic Net-based GTEx v8 eQTL models were downloaded from https://predictdb.org/post/2021/07/21/gtex-v8-models-on-eqtl-and-sqtl/. The summary statistics of GWAS for BMI83, HbA1c levels (total sample based)83, and BMI-adjusted HbA1c levels (total sample based)84 were used to conduct the association analysis. The SNPlocs.Hsapiens.dbSNP144.GRCh37 Bioconductor package85 was used to convert the genomic coordinates of SNPs to rsID for the summary statistics of HbA1c and BMI-adjusted HbA1c. Sensitivity analysis was performed using the CAVIAR fine-mapped variants86 for NK2R in nucleus accumbens of the brain tissue.
Genetic association studies in Greenlanders
Blood samples from Greenlanders collected in three population surveys (data are available at ref. 87 and https://www.sdu.dk/da/sif/rapporter/2011/inuit_health_in_transition and https://www.sdu.dk/da/sif/rapporter/2019/befolkningsundersoegelsen_i_groenland) were genotyped using the Multi-Ethnic Global Array (Illumina). After quality control up to 5,758 individuals were available for association analyses. Metabolic phenotypes were measured as previously described88, and association analyses were performed with a linear mixed model, to account for relatedness and admixture, assuming an additive genetic model and adjusting for age, sex, cohort using GEMMA89. We performed association tests for all 132 variants within the coding region of NK2R and the 3Ⲡand 5Ⲡuntranslated regions ±1,000 bp. The strongest associations across traits related to body composition were observed for the non-coding rs139900276 variant. RNA was extracted from peripheral blood for a subset of 499 individuals. The procedure for RNA extraction, sequencing, quality control, and quantification has previously been described88. NK2R expression according to rs139900276 genotype was tested with a linear mixed model adjusted for sex, age, and top ten principal components from a principal components analysis of the normalized expression matrix.
Mutation of human wild-type NK2R
SNPs identified by GWAS were introduced to human wild-type NK2R coding sequence (NP_001048.2) in a custom pCDNA3.1(+) vector (Genscript) using PCR-based QuickChange Site-Directed Mutagenesis (Agilent) according to manufacturerâs protocol. Mutated vector was introduced to Escherichia coli to amplify DNA and indicated mutations were confirmed by forward and reverse strand Sanger sequencing (Eurofins). PCR primers used to introduce mutations are as follows (forward, reverse): I23T (CAACACCACGGGCACGACAGCCTTCTCCA, TGGAGAAGGCTGTCGTGCCCGTGGTGTTG), V54I (TGACGGGTAATGCCATCATCATCTGGATCATCCTG, CAGGATGATCCAGATGATGGCATTACCCGTCA), A161T (CTGGTGGCTCTCACCCTGGCCTCCC, GGGAGGCCAGGGTGAGAGCCACCAG), R323H (CCGGCTTGCCTTCCATTGCTGCCCATGG, CCCATGGGCAGCAATGGAAGGCAAGCCGG), T346M (CGACCTCCCTCTCCATGAGAGTCAACAGGTG, CACCTGTTGACTCTCATGGAGAGGGAGGTCG), T363A (TGGCTGGGGACGCAGCCCCCTCC, GGAGGGGGCTGCGTCCCCAGCCA), H395R (TTGCCCCCACCAAAACTCGTGTTGAAATTTGAGGATC, GATCCTCAAATTTCAACACGAGTTTTGGTGGGGGCAA).
Receptor activity assays
NKA-induced activation of mutated human NK2R and substance P-, NKA-, NKB-, EB1001- and EB1002-induced activation of mouse and human wild-type NK1R, NK2R and NK3R were measured by inositol-1,4,5-trisphosphate [3H] Radioreceptor assay (IP3 assay). IP3 assays were carried out using COS-7 cells (ATCC, CRL-1651) transiently transfected by calcium phosphate transfection with one of the mutated NK2R variants or the wild-type receptors as previously described75. The IP3 assay was performed the day after transfection. In brief, cells were washed and pre-incubated in assay buffer (HBSS, 10âmM LiCl, 0.2% w/v ovalbumin) for 30âmin followed by 120âmin incubation with substance P, NKA, NKB, EB1001 or EB1002 at 37â°C as indicated in result section and figure legends. After incubation, plates were immediately placed on ice, and cells were lysed (10âmM formic acid). After 30âmin incubation 1âmg per well SPA YSI beads were pipetted into a solid white 96 wells plate and 35âμl of the lysis solution was transferred to the plate. Plates were mixed, briefly centrifuged and left at room temperature for 8âh before counting in a MicroBeta plate counter (Perkin Elmer). Cells have been tested negative for mycoplasma contamination.
Sample size determination and blinding
No statistical methods were applied to predetermine sample size for in vivo pharmacology experiments. Sample sizes were determined on the basis of previous experience with related experimental setups75. Studies were not blinded.
Statistical analysis
Statistical analyses were performed using GraphPad Prism v.9.5.1 or SPSS v.29.0.2.0 (IBM). Sample numbers and statistical analysis methods are provided in the figure legends. Data are presented as meanâ±âs.e.m. unless otherwise specified.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.