The CLOUD experiments
The CERN CLOUD chamber36 was used to conduct the experiments presented in this study. CLOUD is an electropolished, stainless-steel, 26.1-m3 chamber designed to study new particle formation under the full range of tropospheric and lower-stratospheric conditions. The thermal housing around the chamber is able to control the temperature from 208 to 373âK with high precision (±0.1âK)51. CLOUD was operated at a pressure of approximately 965â±â5âmbar in this study. To avoid cross-contamination between different experimental programmes and to achieve extremely low NH3 concentrations, the chamber is cleaned by rinsing the chamber walls with ultrapure water and heating to 373âK for more than 24âh. To maintain cleanliness and ensure minimal contamination, ultrapure synthetic airâderived from mixing cryogenic liquids (21% oxygen and 79% nitrogen)âis continuously injected into the chamber. The chamber is characterized by a low loss rate, with condensation sink values comparable with those observed in pristine environments.
Various light sources are positioned in the CLOUD chamber to selectively drive photochemistry. OH production is initiated by illuminating O3 with a UV fibre-optic system, a combination of four 200-W Hamamatsu Hg-Xe lamps with wavelengths spanning 250 and 450ânm, a krypton fluoride (KrF) excimer UV laser at 248ânm and a 52-W low-pressure mercury lamp centred at 254ânm. As well as O3 photolysis, OH radicals are also produced by photochemical production from nitrous acid (HONO) and hydrogen peroxide (H2O2). Both the HONO and H2O2 generators were designed specifically for CLOUD experiments. Following the same principle as an earlier study52, a gasâliquid mixture of HONO is synthesized from continuous mixing of H2SO4 (Sigma Aldrich, 99%) with sodium nitrite (NaNO2, Sigma Aldrich, 99%) in a stainless-steel reactor53. HONO is transferred from liquid phase to gas phase by flowing nitrogen gas (1â2âlâminâ1) through the reactor. HONO is then introduced into the CLOUD chamber and photolysed by a UV light source centred at 385ânm to produce OH radicals and NO. The HONO reactor is continuously cooled to 5â°C and a cryo-trap is placed between the reactor and the chamber to remove excess water vapour and avoid ice blockage of the chamber input pipe. Gaseous H2O2 is produced from bubbling N2 gas through a H2O2 solution. The H2O2 solution is stored in a glass beaker contained in a stainless-steel container at a constant temperature of 5â°C. A different combination of UV sources is used to photolyse H2O2 to produce different amounts of OH radicals.
A green light sabre centred at 528ânm is used to photolyse molecular iodine (I2). All light systems are continuously monitored by a spectrometer and an array of photodiodes at the bottom of the chamber. Dedicated actinometry experiments allow quantitative determination of actinic fluxes of the light system at different intensities.
Particle formation under different ionization regimes is simulated by combining a strong electric field (±30âkV) and the pion beam produced by the CERN Proton Synchrotron. The electric field eliminates natural ions in less than 1âs, thus creating ion-free conditions (neutral experiments). The pion beam produced by the CERN Proton Synchrotron enhances ion production on top of the galactic cosmic rays. Two magnetically coupled stainless-steel fans mounted at the top and bottom of the chamber enable uniform spatial mixing of particles and vapours within a few minutes. Iodine is injected into the chamber from a temperature-controlled evaporator containing crystalline iodine (I2, Sigma-Aldrich, 99.999% purity) at the bottom of the chamber. The SO2 (Carbagas, 100âparts per million by volume (ppmv) in N2) and isoprene (PanGas, 1,000âppmv in N2) are injected into the chamber from pressurized gas cylinders and the O3 is introduced to the chamber by passing O2 through an ozone generator.
The data presented in this study were collected in two consecutive CLOUD campaigns (CLOUD15 and CLOUD16). The CLOUD15 and CLOUD16 campaigns were carried out from September to November in 2022 and 2023, respectively. Because the experiments reported in this study were carried out at extremely low temperatures (â30â°C and â50â°C), heat-insulation systems (CLOUD15) and active cooling systems (CLOUD16) were used to reduce measurement systematic error. The heat-insulation systems were primarily made with thermal insulation foam to isolate the instrument inlet system from ambient air. The active cooling systems involved circulating the air inside the chamber thermal housing, at the same temperature as the chamber, around the inlet systems of different instruments. The active cooling systems were also wrapped with thermal insulation foam to allow for more effective inlet cooling. These cooling systems were applied to all mass spectrometers and particle counters, except a butanol condensation particle chamber (CPC; TSI 3776), a nano-scanning mobility particle sizer (nano-SMPS, TSI 3938) and a long-SMPS (TSI 3082), which used a heat-insulation system in both campaigns to act as a standard to avoid systematic errors resulting from changing from the heat-insulation system to the active cooling system.
Measurement of chemical composition
Ozone (O3)
O3 was monitored using a gas monitor (Thermo Environmental Instruments, TEI 49C).
Hydroxyl radicals (OH)
The OH radical was measured by HORUS54 (HydrOxyl Radical measurement Unit based on fluorescence Spectroscopy).
Hydroperoxyl radical (HO2)
The HO2 radical was primarily measured using the bromide chemical-ionization mass spectrometer coupled with a multi-scheme chemical-ionization inlet-2 (Br-MION2-CIMS)55 and HORUS in both CLOUD15 and CLOUD16 campaigns. HORUS measures HO2 by chemically converting it to OH by NO. However, the RO2 radical (organic peroxy radicals) produced from isoprene oxidation may also contribute to the HO2 signal measured by HORUS, as the reaction between RO2â+âNO can also produce OH radicals. By contrast, the HO2 measurement by Br-MION2-CIMS is less ambiguous, as it is defined by the peak HO2Brâ (ref.â55). However, the measurement of HO2 by Br-MION2-CIMS is severely affected by airâwater content55, making offline calibration difficult. Therefore, the HO2 measurement by Br-MION2-CIMS was calibrated by HORUS under RO2 radical-free conditions. The online calibration was carried out for every absolute humidity condition reported in this manuscript. During a small section in which the primary ions of Br-MION2-CIMS were saturated by either HONO or H2O2, either the low-pressure bromide chemical-ionization mass spectrometer or HORUS was used to complement the HO2 measurement after intercomparing the data with Br-MION2-CIMS and HORUS during experiments without HONO and H2O2. The precision of OH and HO2 data acquired by the HORUS instrument is quantified at 13% and 7%, respectively, with uncertainties calculated at 1Ï over a 10-min averaging period. Furthermore, the systematic error of the measurement is calculated to be 12% for OH and 30% for HO2.
Nitrogen oxide (NO) and dioxide (NO2)
NO was measured by detecting the chemiluminescence of NO and O3 using a chemiluminescence detector (ECO PHYSICS, CLD 780TR). This instrument was calibrated by a second NO monitor (ECO PHYSICS, CLD 780TR), whichâin turnâwas calibrated using the CMK5 Touch dilution system (Umwelttechnik MCZ GmbH) with a NO bottle (Praxair, 1.00âppmv in N2) and synthetic air (Nippon Gases, hydrocarbon-free). The first detector, which provides data for this study, was found to contain background values that have been subtracted in this study. NO2 was measured by a cavity-attenuated phase-shift nitrogen dioxide monitor (CAPS NO2, Aerodyne Research Inc.). Hourly, the instrument undergoes a 5-min background measurement of pure N2 gas. During the 5-min background measurements, data have been interpolated to give a continuous time series. The NO2 monitor was calibrated using a custom-made cavity-enhanced differential optical absorption spectroscopy instrument56. After the subtraction of an average instrument background concentration, the final NO2 concentration was obtained.
Nitrous acid (HONO) and hydrogen peroxide (H2O2)
Both HONO and H2O2 were detected using bromide chemical-ionization mass spectrometry55, as they exhibit reasonable affinity with the bromide anion. Direct calibrations of these two species were not carried out on-site and the current estimation assumes that they share the same detection sensitivity as H2SO4 (a low-limit estimation). Because these species serve as the precursors of OH and NO radicals, which were reliably traced, the concentrations of HONO and H2O2 are not crucial to the reported results and are therefore omitted from this study.
Two bromide chemical-ionization systems were used to detect HONO and H2O2. The first system, Br-MION2-CIMS, offers sensitive detection of both species at concentrations below about 1010âcmâ3, with a detection limit of around 6âÃâ106âcmâ3 (H2O2) and 1.6âÃâ105âcmâ3 (HONO). However, in some experiments, the estimated HONO and H2O2 concentrations exceeded 1010âcmâ3. The second system, Br-AIM-CIMS, uses bromide chemical-ionization at low pressure in combination with an active water feedback loop to control the Br-hydration in the ion molecule reactor and avoids saturation. Br-AIM-CIMS was used to measure concentrations from above the detection limit of 4.8âÃâ107âcmâ3 (HONO) and 3.3âÃâ107âcmâ3 (H2O2), based on a calibration factor of 3âÃâ1012 for HONO and H2O2.
Sulfur dioxide (SO2)
To measure the concentration of SO2, a gas monitor (Thermo Fisher Scientific Model 42i-TLE) was used. However, as the SO2 concentrations in our experiments were usually below 5âÃâ109âcmâ3 (150âpptv), we also used the Br-MION2-CIMS to measure SO2 (ref.â55) in both CLOUD15 and CLOUD16 campaigns. The measurement of SO2 by Br-MION2-CIMS is substantially affected by airâwater content, so we conducted online SO2 calibration using the SO2 monitor at both â30â°C and â50â°C. The derived calibration factors are 1.7âÃâ1013 at â30â°C and 1.5âÃâ1011 at â50â°C for CLOUD15 and 3.1âÃâ1011 at â50â°C for CLOUD16. During the experiments, when the primary ions of Br-MION2-CIMS were saturated by either HONO or H2O2, the Br-AIM-CIMS was used to complement the SO2 measurement. With an active water sensitivity control, Br-AIM-CIMS measures SO2 concentrations from above the detection limit of 3âÃâ107âcmâ3 with a constant calibration factor of 20âÃâ1012 at â30â°C and â50â°C.
Sulfuric acid (H2SO4)
To ensure the quality of the reported data, we monitored H2SO4 concentrations using two chemical-ionization mass spectrometers: the nitrate chemical-ionization mass spectrometer (NO3-CIMS) and the MION2-CIMS operating in bromide chemical-ionization mode (Br-MION2-CIMS55). Furthermore, isotopically labelled H15NO3 was used during the CLOUD16 campaign to distinguish the nitrogen atom originating from the analyte with the reagent ion. The H2SO4 calibration was carried out by two independent calibration systems. The first set-up used the original calibration box designed by Kürten et al.57 along with their in-house calibration scripts. The second set-up is similar to the original version but with different physical dimensions. Also, the recently developed open-source MARFORCE model is used to simulate H2SO4 production in both calibration set-ups55.
In total, we conducted seven calibration experiments at different stages of the CLOUD15 campaign, and each CIMS instrument was calibrated using both calibration set-ups. Two calibrations were performed for the Br-MION2-CIMS, resulting in equivalent H2SO4 calibration factors of 157% and 149%. For the NO3-CIMS, five calibrations were carried out, resulting in equivalent calibration factors of 88%, 100%, 95%, 154% and 164%. Given that the NO3-CIMS provided most of the H2SO4 concentration in this study, we use the calibration carried out immediately after the experiments for this study. This results in a calibration factor of 6.2âÃâ109âcmâ3 for the NO3-CIMS and an equivalent calibration factor of 9.0âÃâ109âcmâ3 for the Br-MION2-CIMS. We use the minimum and maximum of the seven calibrations, ranging from 88% to 164%, as the systematic error of the H2SO4 detection for CLOUD15. It is important to note that we had to change the optimal inlet flow rates of the Br-MION2-CIMS at â30â°C and â50â°C. The varying temperatures and flow rates result in different inlet loss rates, all of which have been accounted for in this dataset.
As well as the normal H2SO4 calibration, we conducted a set of iodine oxoacid nucleation experiments at â10â°C, similar to those presented in ref.â37. The nucleation rates in these experiments are comparable with all of our earlier experiments, further enhancing our confidence in the reported acid concentrations.
In the CLOUD16 campaign, a total of seven calibration experiments were carried out. Two calibration experiments were conducted for the Br-MION2-CIMS, before and after the presented experiments. The results yield equivalent H2SO4 calibration factors of 120% and 118%. For the labelled NO3-CIMS, six calibrations were performed in total, three before the isoprene experiments, resulting in equivalent calibration factors of 100%, 99% and 88%. It is important to note that, during the last few days of the isoprene experiments, the NO3-CIMS suffered from a pump failure that may have caused a shift (by up to 20%) in the calibration factor owing to a slight change in the sample flow. This potentially affects only two experiments in this study. To correct for this, we have assumed a linear correlation between the sample flow and calibration factor. The failing pumps were then replaced and the data from the rest of the experiments were calibrated after the presented experiments, with two calibrations that yielded equivalent calibration factors of 190% and 185%. This yields a calibration factor of 1âÃâ1010âcmâ3 for the labelled NO3-CIMS and an equivalent calibration factor of 1.9âÃâ1010âcmâ3 for the Br-MION2-CIMS. By considering all of the calibration experiments, the systematic error of H2SO4 detection for CLOUD16 is estimated to range from 88% to 120%. Furthermore, using these two instruments, after applying their respective calibration factors, we compared the measured methanesulfonic acid concentrations from the CLOUD chamber at â50â°C. This comparison demonstrated a good agreement, confirming the accuracy of the calibrations.
Iodine species
We measured iodic acid (HIO3) and iodous acid (HIO2) using both the NO3-CIMS and Br-MION2-CIMS and we use the same calibration factor as H2SO4 in the data analysis, similar to our earlier studies37,45,55,58,59. We used Br-MION2-CIMS to measure I2, which is detected at the collision limit, shown by our recent studies55,60.
Isoprene
Isoprene was measured by a proton transfer reaction mass spectrometer using the hydronium chemical-ionization method61 (H3O-PTR-MS). This particular instrument used in this study is an adapted version, which is explained in greater detail previously62.
ISOPOOH and IEPOX detection and separation
Measuring and distinguishing between ISOPOOH and IEPOX can be experimentally challenging owing to their identical molecular formula (C5H10O3). As a result, mass-spectrometric methods often detect them together at the same exact mass in the same peak35. To address this issue, techniques such as tandem mass spectrometry have been used to separate ISOPOOH and IEPOX from each other29.
In this study, these two isomeric compounds were measured both by the Br-MION2-CIMS and the proton transfer reaction mass spectrometer 3 (ref.â63) operating in ammonium chemical-ionization mode (NH4-PTR3-CIMS64). NH4-PTR3-CIMS measured ISOPOOH and IEPOX primarily as clusters with ammonium cation, as the proton affinity (see the âQuantum-chemical calculationsâ section) of NH3 (204.25âkcalâmolâ1) is higher than that of 1,2-ISOPOOH (198.31âkcalâmolâ1), 4,3-ISOPOOH (195.51âkcalâmolâ1) and cis-β-IEPOX (204.11âkcalâmolâ1). In this study, we also aim to investigate the capability of the Br-MION2-CIMS in detecting ISOPOOH and IEPOX. We calculate the formation free enthalpies of 1,2-ISOPOOH (â27.5âkcalâmolâ1), 4,3-ISOPOOH (â26.9âkcalâmolâ1) and cis-β-IEPOX (â28.0âkcalâmolâ1) with the bromide anion, respectively. We find that the formation free enthalpies are almost equal to the value of hypoiodous acid (HOI) clustered with the bromide anion (26.9âkcalâmolâ1), as presented in ref.â55. Because the instrument used in ref.â55 and in this study is the same and the instrument tuning is identical, the fragmentation of these bromide anion cluster ions should be comparable. He et al.55 calibrated both the H2SO4 and the HOI, and the calibration factor of HOI was approximately two times larger than that of H2SO4. Therefore, the calibration factor used for C5H10O3 is two times the calibration factor for H2SO4 in this study.
As neither the NH4-PTR3-CIMS nor the Br-MION2-CIMS are able to distinguish between ISOPOOH and IEPOX, the reported C5H10O3 in this study is the sum of ISOPOOH and IEPOX. Earlier studies have shown that ISOPOOH is effectively lost to metal surfaces by converting it to methyl vinyl ketone (MVK) and methacrolein (MACR)42,65,66, whereas IEPOX is not affected by metal surfaces67. However, as the experiments in this study focus on extremely low temperatures (â30â°C and â50â°C), the chamber wall itself may also serve as a cryo-trap68 for both ISOPOOH and IEPOX. Therefore, it prevents us from using wall-loss-rate perturbation experiments to separate these two species at these temperatures.
To understand the distribution of ISOPOOH and IEPOX in C5H10O3, we carry out a kinetic simulation using the reduced isoprene oxidation mechanism provided in ref.â35. The results are presented in Extended Data Fig. 1b. The simulation is carried out by the F0AM model43. The model requires input parameters such as isoprene, OH, HO2 and O3 concentrations measured by our instruments.
Another important parameter is the wall-loss rate of IP-OOM. We present an experiment in which we manipulate the loss rate of IP-OOM by turning off the light source and increasing the mixing fan spinning rate from 12% to 100% from the equilibrium conditions in Extended Data Fig. 1a. By turning off the light source, the production of IP-OOM stops. Furthermore, by increasing the fan speed, we increase the maximum wall-loss rate from approximately 1.6âÃâ10â3âsâ1 to 8.5âÃâ10â3âsâ1. The decay rates of C5H10O3 and C5H12O6, with lifetimes of 137âs and 112âs, respectively, are similar to the decay rate of HIO3 (129âs) and also, from previous measurements, H2SO4. Because HIO3 has an accommodation coefficient of unity to the chamber wall, we conclude that C5H10O3 and other species with lower volatilities have similar wall-loss rates. In this study, we apply a general wall-loss rate for these species of 1.6âÃâ10â3âsâ1. This wall-loss rate is calculated from the measured H2SO4 wall-loss rate by correcting the diffusivity of C5H12O6 at â30â°C using the method described by our earlier study58.
We further conduct simulations for all of our experiments using the same procedure, and the ratio of IEPOX in C5H10O3 versus OH concentration is presented in Extended Data Fig. 1b. As anticipated, the IEPOX ratio is positively correlated with OH concentrations. For further analysis, a fit with an expression of ratio of \({10}^{(0.58\times {\log }_{10}([{\rm{OH}}])-4.6)}\) is plotted.
Gas-phase oxidized isoprene products
The gas-phase measurement of IP-OOM was achieved by using a combination of NO3-CIMS, Br-MION2-CIMS and NH4-PTR3-CIMS. As defined in this study, only the species with carbon and oxygen numbers equal to or larger than 4 are considered in the IP0-2N, which are primarily produced from OH oxidation of ISOPOOH and IEPOX with and without involving nitrogen oxides. Furthermore, the particle-phase IP0-2N were monitored by a FIGAERO69, which operates with the bromide chemical-ionization method60 in CLOUD15 (Br-FIGAERO-CIMS) and with the iodide chemical-ionization method in CLOUD16 (I-FIGAERO-CIMS). These chemical-ionization methods exhibit varying preferences for analytes. For example, the NO3-CIMS is renowned for detecting highly oxygenated organic molecules70 that contain more than 5âoxygen atoms. The H3O-PTR-MS is the only one that can detect isoprene, whereas both the NH4-PTR3-CIMS and the Br-MION2-CIMS are capable of detecting semi-volatile organic compounds. Consequently, the combination of these CIMS methods enables the measurement of IP-OOM at different oxidation states.
It is worth mentioning our specialized approach to measuring IP-OOM using Br-MION2-CIMS during experiments involving excess HONO and/or H2O2, as described previously. In these experiments, the primary ions (Brâ and H2OBrâ) were substantially transformed into product ions such as HONOBrâ, H2O2Brâ and (H2O2)2Brâ. Consequently, the measurement of IP-OOM could be compromised if HONO and H2O2 strongly bind with Brâ, thereby impeding the ligand exchange with IP-OOM. Therefore, we extensively compared the Br-MION2-CIMS measurements with those of NO3-CIMS and NH4-PTR3-CIMS during experiments with and without such primary ion saturation to ensure reliable measurements. We found that the Br-MION2-CIMS measurement remained uncompromised when we included HONOBrâ, H2O2Brâ and (H2O2)2Brâ as the primary ions. This is probably because of the relatively weak bonding of HONO and H2O2 with Brâ, which enables effective charging of IP-OOM by allowing ligand exchange reaction. Quantum-chemical calculations further suggest that the formation free enthalpies of HONOBrâ and H2O2Brâ are â23.6 and â21.2âkcalâmolâ1, respectively. These numbers are sufficiently lower than other molecules that are detected at the collision limit by Br-MION2-CIMS55.
To produce IP0-2N, we conducted a set of experiments in which we varied the concentrations of isoprene (ranging from 1.4âÃâ109 to 4.2âÃâ1010âcmâ3) and OH (ranging from 0.1 to 6.9âÃâ107âcmâ3) to alter the distribution of oxidation products35. To analyse the results of these experiments, we present a generic algorithm to calculate the total sum of gaseous IP0-2N produced, with a focus on those with carbon and oxygen numbers greater than 3:
-
1.
IP-OOM are independently identified by each of the CIMS instruments. Their responses to the isoprene oxidation in the chamber are observed to distinguish them from any background contaminations originating from either the chamber or the ion sources. If an individual IP0-2N is affected by contaminants of the same molecular formula, its background, derived from the nearest cleaning stage, is subtracted from its concentrations.
-
2.
If an IP0-2N is detected by only one of the three CIMS, it is added to the total sum directly.
-
3.
If several CIMS detect species with the same molecular formula, their measured signals are compared in pairs to derive a correlation coefficient. A pair is considered to measure identical molecules if the correlation coefficient is greater than 0.5. However, owing to the transfer of the H2SO4 calibration factors to the measured IP0-2N (NO3-CIMS and Br-MION2-CIMS), the concentration of any molecule with a lower detection efficiency than H2SO4 may be underestimated. The extent of this underestimation depends on the chemical-ionization method used, as the binding enthalpies of the analyte-Brâ, analyte-NO3â and analyte-NH4+ may differ. To address this, we add the highest measured concentration of the three CIMS to the IP0-2N and discard the rest, as the highest concentration is probably the closest to the actual concentration.
-
4.
If the correlation coefficient is less than 0.5, we consider that this pair represents two different molecular structures, that is, two isomers or conformers. In this case, both will be added to the IP0-2N.
However, maintaining all three instruments to be operational throughout all experiments presents a challenge, for instance, the Br-MION2-CIMS operated in the APi-TOF mode to measure charged clusters. Therefore, we excluded data collected during periods in which any one of the instruments was not available.
Charged clusters
Naturally charged clusters were measured with two APi-TOF mass spectrometers (Aerodyne Research Inc.) operating at negative and positive ion mode71. The first instrument was equipped with a MION2 operating in the APi-TOF mode (MION2-APi-TOF)55,72 by deactivating the inlet voltages responsible for directing charged reagent ions into the sample flow. The second device was coupled with an ion-molecule reaction chamber (APi-TOF). Overall, the APi-TOF was less sensitive than the MION2-APi-TOF. The charged clusters reported in Fig. 3 were measured with the MION2-APi-TOF, which was validated by the APi-TOF. Because the MION2 inlet was operated in bromide chemical-ionization mode in some experiments, part of the data reported in Extended Data Fig. 7 was measured by the APi-TOF.
Particle-phase measurements
We measured the chemical composition of small particles using a FIGAERO coupled to a chemical-ionization mass spectrometer69. Particles were sampled from the CLOUD chamber onto a 5-µm-pore polytetrafluoroethylene (PTFE) filter (MilliporeSigma). Filter mass loading is dependent on particle distribution in the chamber, collection flow rate (typically 7â8âlâminâ1) and total collection time (1â2âh in this study). After particle collection, the filter was automatically moved to in front of the ion molecule reactor. The filter aligned with a sealed port that constantly flushes pure N2. In CLOUD15, the flow rate during chemical measurement was 3âlâminâ1 and it was increased to 5âlâminâ1 in CLOUD16 for more efficient heat transfer in a longer port. Pure N2 was heated from room temperature up to 180â°C using programmed thermal desorption controlled by eyeon software v2.1.4.5. As the filter temperature increased, we detected lower-volatility molecules partitioning back into the gas phase. For the particle filter loadings in this study, we observed that all signals decreased back to the baseline by the end of the heating cycle, indicating no notable remaining mass.
Typically, FIGAERO-CIMS is operated using Iâ chemical-ionization in a reduced-pressure ion molecule reactor (about 120â150âmbar). Pure N2 is flowed around a CH3I permeation tube (Vici) and through a 210Po ionizer (NRD LLC) to produce iodide ions. These polarizable ions effectively form adducts with oxygenated organic compounds, with a small fraction of interactions leading to charge transfer between the ion and neutral compound. In CLOUD15, we used Brâ chemical-ionization to distinguish between our chemical-ionization reagent and iodine species inside the CLOUD chamber. The set-up is the same as iodide ionization mode except we exchange a CH2Br2 permeation tube and heat it to 40â°C to increase permeation rates. These chemical-ionization techniques are both sensitive to oxygenated organic compounds, organics with nitrate and sulfate functional groups and inorganic acids60,69. Compounds chemically transformed through deprotonation or thermal decomposition have been excluded, as their parent molecule is unknown.
Particle number size distribution
The Neutral cluster and Air Ion Spectrometer73,74 (NAIS) was used to measure the naturally charged particle number size distribution from 0.8 to 41ânm and the particle number size distribution (naturally chargedâ+âneutral) from 2 to 42ânm in both negative and positive polarities. The nano-condensation nucleus counter was used to measure the particle number size distribution between 1 and 3ânm. It consists of a particle size magnifier75 (PSM, Airmodus Oy). The PSM, which is an aerosol pre-conditioner, uses diethylene glycol to grow aerosol particles as small as 1ânm to sizes that can be easily detected by a CPC75. Furthermore, a butanol CPC (TSI 3776) was used to measure the total number concentration of particles with diameters greater than 2.5ânm. A nano-scanning mobility particle sizer (TSI 3938)76 coupled to a butanol CPC (TSI 3776), was used to measure the particle-size distribution within the range 6â65ânm, whereas particles larger than 65ânm were measured using a commercially available long-SMPS (TSI 3082) coupled to a water butanol CPC (TSI 3775).
Yield of IP0N from ISOPOOH
As shown in a previous section, the IP0N in this study is defined as species with C,Oââ¥â4. Therefore, ISOPOOH and IEPOX are not included in the IP0N. ISOPOOH and IEPOX are treated as the direct precursors of IP0N, which in turn contribute to isoprene new particle formation. It is worth noting that both ISOPOOH and IEPOX undergo oxidation, producing compounds with C,Oââ¥â4. However, the reaction rate of ISOPOOH is approximately ten times larger than IEPOX35. To account for the difference in reaction-rate coefficients, we predict the ratio of IEPOX in C5H10O3 using the data shown in Extended Data Fig. 1b based on the OH concentrations. Assuming that the concentration of IP0N is at equilibrium, the primary mechanism for IP0N loss is wall deposition, which is approximately equal to the production of IP0N from ISOPOOH and IEPOX. Therefore,
$$\begin{array}{l}[{{\rm{IP}}}_{0{\rm{N}}}]\times {k}_{{\rm{wall}}}\,=\,R\times ({k}_{{\rm{OH}} \mbox{-} {\rm{ISOPOOH}}}\times [{\rm{OH}}]\times [{\rm{ISOPOOH}}]\\ \,\,\,\,\,\,+{k}_{{\rm{OH}} \mbox{-} {\rm{IEPOX}}}\times [{\rm{OH}}]\times [{\rm{IEPOX}}])\end{array}$$
in which kOH-ISOPOOH and kOH-IEPOX are the reaction-rate coefficients of ISOPOOH (10â10âcm3âsâ1) and IEPOX (10â11âcm3âsâ1) with OH (ref.â35), respectively; [IP0N], [OH], [ISOPOOH] and [IEPOX] show concentrations and kwall is the wall-loss rate of C5H12O6; R represents the yield of IP0N from C5H10O3.
We then define the reacted C5H10O3 (cmâ3) as:
$${\rm{Reacted}}\,{{\rm{C}}}_{5}{{\rm{H}}}_{10}{{\rm{O}}}_{3}=\frac{{k}_{\text{OH-ISOPOOH}}\times [{\rm{OH}}]\times [{\rm{ISOPOOH}}]+{k}_{\text{OH-IEPOX}}\times [{\rm{OH}}]\times [{\rm{IEPOX}}]}{{k}_{{\rm{wall}}}}$$
The yield of IP0N from reacted C5H10O3 is depicted in Extended Data Fig. 2. We find that the yields of IP0N are approximately 46% at â30â°C and 55% at â50â°C. However, it is essential to note that the detection of C5H10O3, IP0N and OH has various uncertainties. We estimate that the derived yield has an uncertainty of at least a factor of two, with the quantification of IP0N being the main source of uncertainty.
One further source of error in determining the yield is the contribution of highly oxygenated molecule production from the first-generation isoprene hydroxy peroxy radical (ISOPOO, C5H9O3) through auto-oxidation or dimer formation. For example, the reaction between two ISOPOO radicals can generate C10H18O4, and intramolecular H-shift followed by HO2 termination of ISOPOO produces C5H10O5. Although these two molecules only contribute to a small fraction of IP0N in this study, other similar channels may contribute to a greater extent to IP0N, thereby reducing the yield of IP0N from C5H10O3. As disentangling first-generation and second-generation highly oxygenated molecules from isoprene oxidation is not the objective of this study, future research is necessary to investigate this direction.
Quantum-chemical calculations
Quantum-chemical methods are used to compute cluster formation free enthalpies and proton affinities. Initially, the Spartanâ18 program is used for the conformational sampling with the MMFF method. Subsequently, density function theory (DFT) methods are used to optimize the molecules first at the B3LYP/6-31+G(d) level of theory, followed by optimization and frequency calculations at the ÏB97X-D/aug-cc-pVTZ-PP level of theory77,78 on conformers within 2âkcalâmolâ1 in relative electronic energies. Bromine pseudopotential definitions are obtained from the Environmental Molecular Sciences Laboratory (EMSL) basis set library79,80. The DFT calculations are carried out using the Gaussian 16 program81. To refine the DFT-calculated enthalpies, an extra coupled-cluster single-point energy correction is performed at the DLPNO-CCSD(T)/def2-QZVPP level of theory on the lowest-energy conformers. This coupled-cluster calculation is conducted using the ORCA program version 5.0.3 (ref.â82).
Calculation of the nucleation and growth rates
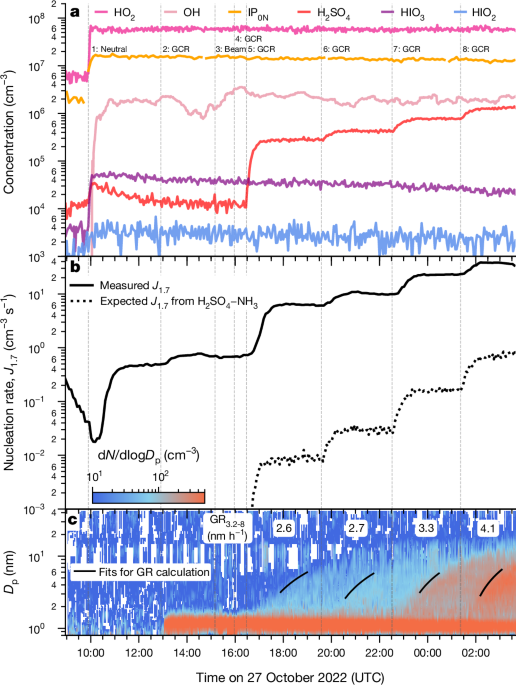
The nucleation rate, J1.7, is calculated on the basis of PSM measurement of particles at a mobility diameter of 1.7ânm (1.4ânm in physical diameter83), which are generally considered to be larger than their critical cluster sizes and thus stable.
To determine the nucleation rates, the time evolution of the particle concentration is analysed, taking into account various loss processes that also affect the concentration. However, because loss processes in a chamber setting differ from those in the atmosphere, the calculation method must be adjusted for chamber experiments84. Specifically, the nucleation rate (J1.7) is calculated by factoring in losses specific to the CLOUD chamber, such as dilution, wall and coagulation losses. We calculated Jdp as follows:
$${J}_{1.7}=\frac{{\rm{d}}N}{{\rm{d}}t}+{S}_{{\rm{dil}}}+{S}_{{\rm{wall}}}+{S}_{{\rm{coag}}}$$
in which dN/dt is the time derivative of the total particle concentration above a certain particle size (here >1.7ânm for J1.7) and Sdil, Swall and Scoag are the particle losses owing to dilution, wall and coagulation. The details can be found in ref.â84. To calculate the coagulation sink, we used the combined particle-size distribution from three instruments (NAIS, nano-SMPS and long-SMPS).
Furthermore, the nucleation rate at 2.5ânm, J2.5, derived from the butanol CPC and corrected by the same method described above, is calculated. The results are presented in Extended Data Fig. 6 in the same format as in Fig. 2. Because the CPC was not affected by the systematic upgrade in the cooling system between CLOUD15 and CLOUD16, it serves to distinguish subtle changes in our data. For example, the nucleation rates from experiments with NOx (filled squares in Fig. 2) seem to be similar to the experiments without NOx (filled circles in Fig. 2). This is probably a result of systematic errors introduced by changing either the cooling system or the instrument-calibration experiments. On the other hand, Extended Data Fig. 6 shows that experiments with NOx have nucleation rates higher than the experiments without NOx, therefore, isoprene nitrates (IP1-2N) do contribute, despite to a lesser extent compared with IP0N, to particle nucleation.
To calculate particle growth rates, we use the 50% appearance-time method, as outlined in previous studies58,84,85. It is worth noting that the appearance-time method can overestimate growth rates when the impacts of coagulation (coagulation sink, coagulation source and particle coagulation growth) are non-negligible compared with the condensation growth, but the coagulation impact is rather small in the CLOUD experiments. For a deeper understanding of the molecular-level theory behind the method, we refer to the theoretical derivation presented in ref.â58. The particle number size distribution data used to calculate growth rates between 3.2 and 8.0ânm are measured by the NAIS. During previous experiments with α-pinene and sulfuric acid, we have confirmed that the growth rates measured with the NAIS in total mode are similar to those measured with the DMA-train86.
Comparison of experimental and ambient conditions
To compare the CLOUD experimental conditions with ambient conditions in the tropical upper troposphere, we summarize in Extended Data Table 1 the key chemical and physical parameters of the CLOUD experiments and the CAFE-Brazil (CB) flight campaign13. The CLOUD statistics are summarized from all experiments presented in this study, separated into two temperature conditions at â30â°C and â50â°C, respectively. Statistics of the CB flight campaign are derived from a single research flight, RFâ19, samples T4 and T9, shown in Fig. 1 of ref.â13. All vapour concentrations are presented in units of molecules per cmâ3âthe quantities as measuredâfor both CLOUD and CB. The values from the CB campaign are not corrected to their values at standard temperature and pressure, to allow for a direct comparison of the chemical and aerosol formation kinetics between the CLOUD experiments and the flight measurements. We elaborate below on three aspects of this comparison: (1) isoprene non-nitrates (IP0N) and nitrates (IP1-2N); (2) atmospheric acids; and (3) impact of atmospheric pressure on particle nucleation.
Distribution of IP0N and IP1-2N
In general, the CLOUD experiments were designed to mimic ambient conditions as closely as possible. Key parameters such as temperature, relative humidity (RH), isoprene, O3, NO and HO2/OH ratios are directly comparable between the CLOUD experiments and the CB measurements. The largest differences between CLOUD and CB are higher atmospheric pressure in CLOUD and higher OH/HO2 concentrations, resulting in a higher HO2/NO ratio in CLOUD. The higher OH concentration in the CLOUD chamber is required to reproduce ambient IP-OOM concentrations at a chamber-wall-loss rate of approximately 2âÃâ10â3âsâ1. In the upper troposphere, the condensation sink for low-volatility gaseous species could be several times or even up to one order of magnitude lower than the chamber-wall-loss rate.
Because this study aims to investigate the contribution of IP0N and IP1-2N to particle nucleation and growth, these parameters are critical for CLOUD to reproduce at atmospheric concentrations. A consequence of the relatively higher HO2/NO ratio in CLOUD is that the IP0N to IP1-2N ratio is elevated compared with CB. However, because the higher operating pressure in CLOUD favours the formation of IP1-2N by accelerating the reaction of organic peroxy radical (RO2) with NO as well as enhancing the organic nitrate formation branching ratio35 (see Extended Data Table 1), this effect largely compensates for the higher HO2/NO ratio in CLOUD. Nevertheless, regardless of the chemical details, which will be presented by follow-up studies, CLOUD successfully reproduces isoprene oxidation products IP0N and IP1-2N, in terms of both absolute values and relative ratios (Extended Data Table 1). The wide range of IP0N and IP1-2N concentrations and IP0N/IP1-2N ratios covered by CLOUD experiments enables CLOUD to reasonably simulate particle nucleation and growth dynamics, consistent with CB measurements during dawn hours (T4 period in Extended Data Table 1) and morning (T9 period in Extended Data Table 1). As the daylight hours proceed beyond the period measured by CB, both OH and HO2 concentrations will increase, whereas NOx concentrations decrease, favouring the formation of IP0N over IP1-2N. Thus, the importance of IP0N may be further enhanced after noon. It is also noteworthy that chemical distribution and nucleation dynamics might differ in other seasons and locations from those covered by CB measurements, such as the cases presented in Fig. S11 of ref.â5. Therefore, we believe that the wide range of conditions explored by CLOUD provides valuable data to enable global models to evaluate the impact of isoprene on new particle formation in other upper-tropospheric environments in which NOx concentrations may differ from those measured by CB.
Impact of sulfuric acid and iodine oxoacids
In this study, we observe a large enhancement of IP-OOM nucleation rates from trace amounts of atmospheric acids (specifically, H2SO4 and HIOx). The enhancement starts at acid concentrations of 105âcmâ3 and, at an acid concentration of 2âÃâ106âcmâ3, the particle-nucleation rate is approximately 100-fold faster than without added acids.
The CB measurements are unable to comment on this synergistic role of acids for IP-OOM particle nucleation owing to their H2SO4 detection limit of several times 106âcmâ3. Nevertheless, acid enhancement of IP-OOM nucleation can be expected to occur in the atmosphere. Aircraft measurements indicate that approximately 10âpptv SO2 is ubiquitous in the global atmosphere between the marine boundary layer and the upper troposphere87,88. Global simulations also suggest that H2SO4 concentrations greater than 105âcmâ3 are widespread throughout the troposphere44. The global distribution of HIOx is less well known and global simulations and aircraft measurements are needed to quantify its concentrations in the upper troposphere. However, recent measurements of iodine oxide and particle-phase iodine in the upper troposphere89 suggest that HIOx may also play a role in enhancing IP-OOM particle nucleation.
Impact of atmospheric pressure on particle nucleation
Cluster-forming interactions (such as nucleation) are of the form:
$$\begin{array}{ll}{\rm{A}}+{\rm{B}}\to {{\rm{C}}}^{* } & ({\rm{R}}1\,;\,{\rm{condensation}}),\\ {{\rm{C}}}^{* }\to {\rm{A}}+{\rm{B}} & ({\rm{R}}2\,;\,{\rm{evaporation}})\,{\rm{and}}\\ {{\rm{C}}}^{* }+{\rm{M}}\to {\rm{C}}+{\rm{M}} & ({\rm{R}}3\,;\,{\rm{thermalization}}),\end{array}$$
in which A and B are two molecules (or small clusters) forming the cluster C and C* is a vibrationally excited state of the cluster containing the cluster energy EAB as part of its internal vibrational energy. The excited cluster C* will lose energy to the bath gas, M, with a concentration given by pressure through the ideal gas law. Cluster-forming interactions can therefore depend on ambient pressure90. When pressure is relatively âlowâ, reaction R3 will be the rate-limiting step for cluster formation and the overall rate will be of the third order (in A, B and M). However, when pressure is relatively high, reaction R1 will be the rate-limiting step and the rate will be of the second order (in A and B) and be independent of pressure, that is, at the so-called âhigh-pressure limitâ. The critical pressure occurs when the rates of R2 and R3 are equal and therefore depends on the lifetime (evaporation rate) of C* through reaction R2, as well as the ambient pressure through reaction R3.
In practice, only systems with very few heavy atoms (four or fewer heavy atoms, in which âheavyâ excludes hydrogen) would show any meaningful pressure dependence in the atmosphere91, as quantified below. Because IP-OOM nucleation typically involves more than 15 heavy atoms, measurements in the CLOUD chamber at near 1âbar are directly applicable to the upper troposphere near 0.2âbar, provided the results are interpreted in terms of number concentrations (thus accounting for the dilution effect of reduced pressure) and not mixing ratios.
The (microcanonical) decomposition rates (inverse lifetimes) of the cluster are given by RiceâRamspergerâKasselâMarcus theory90. It depends strongly on the number of internal vibrational modes in C*. As a rule, it can be estimated (by Rice, Ramsperger and Kassel theory)90 roughly as the âfractional excess free energyâ, \(\upsilon {\left(\frac{e-{e}_{0}}{e}\right)}^{s}\), in which Ï is a typical frequency, 100âTHz or so, e is the cluster energy above the ground vibrational state and e0 is the critical energy for decomposition (the cluster energy EAB) and s is an effective number of vibrational modes in the cluster. Approximately, eâââe0 will be on the order kT (200âcmâ1), whereas for the systems nucleating under atmospheric conditions, e0 will be on the order 800âcmâ1 or more. Thus, e will be on the order 1,000âcmâ1 and (eâââe0)/e will be on the order 0.2. Again, approximately and conservatively, s is 3Nâââ7, in which N is the number of heavy (non-H) atoms in C*. The â7â excludes external modes as well as the reaction coordinate. A system with five heavy atoms would have a decay coefficient of roughly 2.6âÃâ108âsâ1, whereas the collision frequency at 1âatm is near 1010âsâ1. Such a system would (barely) show some pressure dependence. By contrast, for isoprene oxidation products and H2SO4, N is probably 15, so sâ=â3Nâââ7â=â38. Given this, the microcanonical decay coefficients for these clusters will be on the order 3âÃâ10â13âsâ1. This is extremely slow. In practice, it means that the energy distribution in cluster C will be entirely thermal, that is, given by a Boltzmann term at the ambient temperature, and the rates of cluster formation (and decomposition or evaporation) will be unaffected by pressure.