Credit: Steve Russell/Toronto Star via Getty
Colombian neurologist Francisco Lopera, who has died aged 73, changed the course of research on Alzheimer’s disease — both with insights into its mechanism and with his personal, family-centred, long-term approach. He met with thousands of people in a single family, parsing their stories to track hereditary dementia. This level of doctor–patient trust became the foundation for a clinical trial in which, for the first time, a single, extensive kindred agreed to participate. He identified a mutation responsible for early-onset Alzheimer’s disease. The size of the family also created the opportunity to detect, in a small number of family members, rare protective genes that can delay the disease onset by decades.
In the vast majority of Alzheimer’s cases, the cause remains unknown. Cases linked to single mutations are rare — but most of what we know about the disease stems from them. By finding such a mutation, present at birth and leading to dementia some 45 years later, Lopera and his colleagues contributed to the hunt for the disease’s biochemical pathways. They also made progress towards a treatment for the disease, which affects more than 10% of people over the age of 65 and is among the leading causes of death globally.
Can flashing lights stall Alzheimer’s? What the science shows
Lopera was born in the small town of Aragón in northwest Colombia, and trained in medicine at the nearby University of Antioquia in the provincial capital Medellín, where he spent his entire career. At the time, newly qualified doctors had to complete a year of obligatory social service, called rural. Lopera spent it near the border with Panama, where he encountered unusual situations such as vampire bats spreading rabies in children.
In 1982, entering neurology practice in Medellín and always curious about unusual cases, he came across a 47-year-old man with mid-life dementia — a disease present in several generations of the man’s family. For a decade, Lopera and his colleague, psychologist Lucia Madrigal, travelled through the countryside of Antioquia and deep into the barrios (districts) of Medellín, where they heard repeatedly of early-onset memory loss affecting people who, it would turn out, were part of the same family. They assembled a vast family tree, which revealed that, on average, 50% of an affected parent’s offspring developed the disease. This pattern strongly suggested that a mutated gene was responsible.
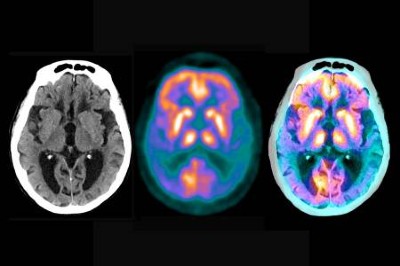
In 1989, I met Lopera in Bogotá. He showed me the family tree and we instantly began a collaboration that would last for 35 years. Without post-mortem investigation, the cause of the memory loss was unknown. With some difficulty, Lopera persuaded a family to allow such an investigation. A neuropathologist flew to my house in Boston, Massachusetts, with the brain of an affected member of the family who had died. We observed the hallmarks of Alzheimer’s disease — deposits of amyloid-β protein (senile plaques) and tau-protein aggregates inside neurons (neurofibrillary tangles). Lopera went on to establish an invaluable brain bank in Medellín that today has more than 500 brains.
Conquering Alzheimer’s: a look at the therapies of the future
Throughout the 1980s and early 1990s, many family members contributed blood samples. Despite the unreliable electricity supply during the years of civil war in Colombia, enough DNA was extracted to pinpoint a mutation in the presenilin gene. It came to be known as the paisa mutation, after the people who live in the region. One woman carried the mutation but did not have dementia even into her mid-seventies, when she died of a melanoma. An imaging study revealed that her brain was loaded with senile plaques but had very few neurofibrillary tangles. Clearly her resilience to dementia and paucity of tangles, despite her large amyloid burden, pointed to protective mechanisms at work.
Genetic diagnosis allowed carriers to be identified before the development of symptoms, raising the possibility of delaying or preventing them in a clinical trial targeting the underlying pathology. But would the families agree to participate? In an area where witchcraft, known locally as brujería, held more sway than the medical system — and where people stigmatized the disease as bobera, or ‘madness’ — Lopera gently presented the logic and evidence of biomedical science. The families’ trust and confidence in him led to high enrolment and the lowest dropout rate of any Alzheimer’s trial so far. Unfortunately, as is often the case with this disease, the drug did not show efficacy. But Lopera’s approach facilitated clinical trials for the next generation of drugs. It also put Colombian neuroscience at the forefront of research into neurodegeneration.
Lopera’s research team has since discovered numerous rare mutations that cause neurodegenerative conditions, some affecting large families. The admixture of the Spanish, African and Indigenous populations in Colombia over the past 500 years has created enormous genetic diversity, with large segments of DNA from each ancestry spread throughout the population. After the Spanish conquistadores arrived in the Americas in 1492, the Indigenous population was decimated by disease. Some of those who survived carried rare mutations that differed in each region of the country, even in each small town. As populations rapidly expanded, so did the frequency of the local rare mutations. These mini-bottlenecks have resulted in a genetic disease map that corresponds to the geographical map.
Lopera’s approach was built on his philosophy: “They don’t come to us; we go to them.” It produced good science and goodwill, opening the way for new clinical trials and the discovery of rare protective variants.
Competing Interests
The author declares no competing interests.