Sato, S. & Kiyono, H. The mucosal immune system of the respiratory tract. Curr. Opin. Virol. 2, 225–232 (2012).
Kiyono, H. & Azegami, T. The mucosal immune system: from dentistry to vaccine development. Proc. Jpn Acad. B 91, 423–439 (2015).
Kiyono, H., Bienenstock, J., McGhee, J. R. & Ernst, P. B. The mucosal immune system: features of inductive and effector sites to consider in mucosal immunization and vaccine development. Reg. Immunol. 4, 54–62 (1992).
Rusell, W. R. & Ogra, R. L. in Mucosal Vaccines—Innovation for Preventing Infections Diseases, 2nd edn (eds Kiyono, H. & Pascual, D. W.) 3–17 (Academic Press, 2020). This chapter provides a comprehensive summary of the history of research and development of mucosal vaccines including those of nasal vaccines.
Okuno, Y. & Nakamura, K. Prophylactic effectiveness of live influenza vaccine in 1965. Biken J. 9, 89–95 (1966).
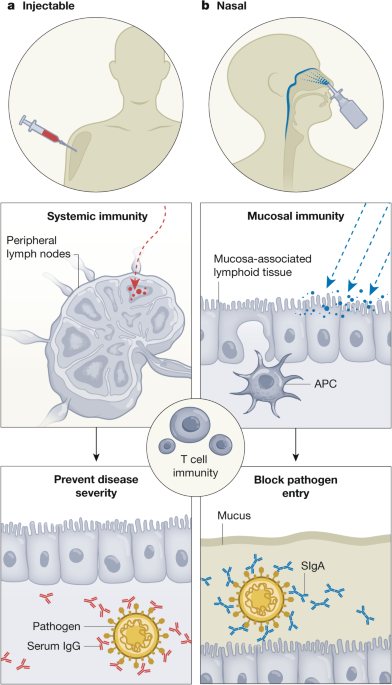
Kiyono, H. & Fukuyama, S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat. Rev. Immunol. 4, 699–710 (2004).
Turner, J. S. et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 596, 109–113 (2021).
Bleier, B. S., Ramanathan, M. Jr & Lane, A. P. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol. Head Neck Surg. 164, 305–307 (2021).
Tiboni, M., Casettari, L. & Illum, L. Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines? Int. J. Pharm. 603, 120686 (2021).
Azzi, L. et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. eBioMedicine 75, 103788 (2022).
Tang, J. et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol. 7, eadd4853 (2022).
Yuki, Y. & Kiyono, H. Mucosal vaccines: novel advances in technology and delivery. Expert Rev. Vaccines 8, 1083–1097 (2009).
Kiyono, H., Yuki, Y., Nakahashi-Ouchida, R. & Fujihashi, K. Mucosal vaccines: wisdom from now and then. Int. Immunol. 33, 767–774 (2021).
Teijaro, J. R. et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187, 5510–5514 (2011).
Diallo, B. K. et al. Intranasal COVID-19 vaccine induces respiratory memory T cells and protects K18-hACE mice against SARS-CoV-2 infection. NPJ Vaccines 8, 68 (2023).
Uddback, I. et al. Prevention of respiratory virus transmission by resident memory CD8+ T cells. Nature 626, 392–400 (2024).
Holmgren, J. & Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 11, S45–S53 (2005).
Dhama, K. et al. COVID-19 intranasal vaccines: current progress, advantages, prospects, and challenges. Hum. Vaccin. Immunother. 18, 2045853 (2022).
Nakahashi-Ouchida, R., Fujihashi, K., Kurashima, Y., Yuki, Y. & Kiyono, H. Nasal vaccines: solutions for respiratory infectious diseases. Trends Mol. Med. 29, 124–140 (2023).
Tsai, C. J. Y., Loh, J. M. S., Fujihashi, K. & Kiyono, H. Mucosal vaccination: onward and upward. Expert Rev. Vaccines 22, 885–899 (2023).
Gizurarson, S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr. Drug Deliv. 9, 566–582 (2012).
Mitchison, H. M. & Valente, E. M. Motile and non-motile cilia in human pathology: from function to phenotypes. J. Pathol. 241, 294–309 (2017).
Voynow, J. A. & Rubin, B. K. Mucins, mucus, and sputum. Chest 135, 505–512 (2009).
Gizurarson, S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 38, 497–506 (2015).
Pabst, R. Nose-associated lymphoid tissue (NALT)—structure, function and species differences. Vaccine 33, 4406–4413 (2015). This review introduces and discusses the basic immunological basis of the NALT as the inductive site for the initiation of antigen-specific immune responses, including species differences.
Ramirez, S. I. et al. Immunological memory diversity in the human upper airway. Nature 632, 630–636 (2024). This study provided evidence that the human nasopharynx, including adenoids, harbours an immunologic environment for IgA B cell induction.
Corr, S. C., Gahan, C. C. & Hill, C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 52, 2–12 (2008).
Stavnezer, J. & Kang, J. The surprising discovery that TGFβ specifically induces the IgA class switch. J. Immunol. 182, 5–7 (2009).
Carrasco-Yepez, M. M. et al. Naegleria fowleri immunization modifies lymphocytes and APC of nasal mucosa. Parasite Immunol. 40, e12508 (2018).
Wellford, S. A. et al. Distinct olfactory mucosal macrophage populations mediate neuronal maintenance and pathogen defense. Mucosal Immunol. 17, 1102–1113 (2024).
Liu, J. et al. Turbinate-homing IgA-secreting cells originate in the nasal lymphoid tissues. Nature 632, 637–646 (2024). This article provides evidence for the existence of a common mucosal immune system through the adoptive transfer of lymphoid B cells leading to their migration to distant and different mucosal tissues.
Nakahashi-Ouchida, R., Yuki, Y. & Kiyono, H. Development of a nanogel-based nasal vaccine as a novel antigen delivery system. Expert Rev. Vaccines 16, 1231–1240 (2017). This review describes how NALT-derived IgA+ B cells predominantly express CCR10 and migrate to immunological effector tissues with increased CCL28 expression.
Hieshima, K. et al. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J. Immunol. 173, 3668–3675 (2004).
Wang, J. et al. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 211, 2397–2410 (2014).
McDermott, M. R. & Bienenstock, J. Evidence for a common mucosal immunologic system: I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 122, 1892–1898 (1979). This paper provided the evidence for the existence of the commensal mucosal immune system.
Wellford, S. A. et al. Mucosal plasma cells are required to protect the upper airway and brain from infection. Immunity 55, 2118–2134.e2116 (2022).
Moens, L. & Tangye, S. G. Cytokine-mediated regulation of plasma cell generation: IL-21 takes center stage. Front. Immunol. 5, 65 (2014).
Corthesy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 4, 185 (2013). This article summarizes the immunobiological uniqueness of SIgA antibodies, which have crucial roles in creating a balanced state of mutualism and elimination in the harsh environment of mucosal surfaces.
Wei, H. & Wang, J. Y. Role of polymeric immunoglobulin receptor in IgA and IgM transcytosis. Int. J. Mol. Sci. 22, 2284 (2021).
Brandtzaeg, P. Secretory IgA: designed for anti-microbial defense. Front. Immunol. 4, 222 (2013).
Miyamoto, S. et al. Infectious virus shedding duration reflects secretory IgA antibody response latency after SARS-CoV-2 infection. Proc. Natl Acad. Sci. USA 120, e2314808120 (2023). This paper demonstrated the importance of SARS CoV-2 S protein-specific SIgA antibodies in preventing infectious virus shedding and transmission in humans.
Marcotte, H. et al. Conversion of monoclonal IgG to dimeric and secretory IgA restores neutralizing ability and prevents infection of Omicron lineages. Proc. Natl Acad. Sci. USA 121, e2315354120 (2024).
Kim, D. Y. et al. The airway antigen sampling system: respiratory M cells as an alternative gateway for inhaled antigens. J. Immunol. 186, 4253–4262 (2011).
Lee, H. et al. Phenotype and function of nasal dendritic cells. Mucosal Immunol. 8, 1083–1098 (2015).
Tamura, S., Tanimoto, T. & Kurata, T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J. Infect. Dis. 58, 195–207 (2005).
Oh, J. E. et al. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci. Immunol. 6, eabj5129 (2021). This paper showed that intranasal, but not injection, immunization induces lung-resident IgA B cells, leading to the induction of IgA-mediated protective immunity against influenza virus.
Treanor, J. J. et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 18, 899–906 (1999).
Thwaites, R. S. et al. Early mucosal events promote distinct mucosal and systemic antibody responses to live attenuated influenza vaccine. Nat. Commun. 14, 8053 (2023).
Ali, M. S. & Pearson, J. P. Upper airway mucin gene expression: a review. Laryngoscope 117, 932–938 (2007).
Dehghan, M. H., Gaikwad, V. M. & Dandge, B. Nasal absorption of drugs—barriers and solutions. Res. J. Pharm. Tech. 2, 634–641 (2009).
Singh, A. K., Singh, A. & Madhv, N. V. S. Nasal Cavity, a promising transmucosal platform for drug delivery and research approaches from nasal to blain targetting. J. Drug Delivery https://doi.org/10.22270/jddt.v2i3.163 (2012).
Robert-Guroff, M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 18, 546–556 (2007).
Yahalom-Ronen, Y. et al. A single dose of recombinant VSV-∆G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 11, 6402 (2020).
Bezbaruah, R. et al. Developmental landscape of potential vaccine candidates based on viral vector for prophylaxis of COVID-19. Front. Mol. Biosci. 8, 635337 (2021).
Ura, T., Okuda, K. & Shimada, M. Developments in viral vector-based vaccines. Vaccines 2, 624–641 (2014).
Spearman, P. et al. Intranasal parainfluenza virus type 5 (PIV5)-vectored RSV vaccine is safe and immunogenic in healthy adults in a phase 1 clinical study. Sci. Adv. 9, eadj7611 (2023).
Ding, C., Ma, J., Dong, Q. & Liu, Q. Live bacterial vaccine vector and delivery strategies of heterologous antigen: a review. Immunol. Lett. 197, 70–77 (2018).
LeCureux, J. S. & Dean, G. A. Lactobacillus mucosal vaccine vectors: immune responses against bacterial and viral antigens. mSphere 3, e00061–18 (2018).
Li, L. et al. Mucosal IgA response elicited by intranasal immunization of Lactobacillus plantarum expressing surface-displayed RBD protein of SARS-CoV-2. Int. J. Biol. Macromol. 190, 409–416 (2021).
Sakurai, F., Tachibana, M. & Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 42, 100432 (2022).
De Leo, V., Milano, F., Agostiano, A. & Catucci, L. Recent advancements in polymer/liposome assembly for drug delivery: from surface modifications to hybrid vesicles. Polymers 13, 1027 (2021).
Mangla, B. et al. Nanocarriers-assisted needle-free vaccine delivery through oral and intranasal transmucosal routes: a novel therapeutic conduit. Front. Pharmacol. 12, 757761 (2021).
Schwendener, R. A. Liposomes as vaccine delivery systems: a review of the recent advances. Ther. Adv. Vaccines 2, 159–182 (2014).
Zuglianello, C. & Lemos-Senna, E. The nanotechnological approach for nasal delivery of peptide drugs: a comprehensive review. J. Microencapsul. 39, 156–175 (2022).
Yuki, Y. et al. Nanogel-based antigen-delivery system for nasal vaccines. Biotechnol. Genet. Eng. Rev. 29, 61–72 (2013).
Nakahashi-Ouchida, R., Yuki, Y. & Kiyono, H. Cationic pullulan nanogel as a safe and effective nasal vaccine delivery system for respiratory infectious diseases. Hum. Vaccin. Immunother. 14, 2189–2193 (2018).
Shimizu, T. et al. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem. Biophys. Res. Commun. 367, 330–335 (2008).
Ayame, H., Morimoto, N. & Akiyoshi, K. Self-assembled cationic nanogels for intracellular protein delivery. Bioconjug. Chem. 19, 882–890 (2008).
Nochi, T. et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 9, 572–578 (2010). This is the first study to demonstrate that a nanogel consisting of cCHP is a promising nasal vaccine-delivery vehicle for universal protein-based vaccines.
Kong, I. G. et al. Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae. Infect. Immun. 81, 1625–1634 (2013).
Fukuyama, Y. et al. Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques. Mucosal Immunol. 8, 1144–1153 (2015).
Nakahashi-Ouchida, R. et al. A nanogel-based trivalent PspA nasal vaccine protects macaques from intratracheal challenge with pneumococci. Vaccine 39, 3353–3364 (2021).
Nakahashi-Ouchida, R. et al. Induction of mucosal IgA-mediated protective immunity against nontypeable Haemophilus influenzae infection by a cationic nanogel-based P6 nasal vaccine. Front. Immunol. 13, 819859 (2022).
Crain, M. J. et al. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58, 3293–3299 (1990).
Nabors, G. S. et al. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18, 1743–1754 (2000).
Piao, Z. et al. Protective properties of a fusion pneumococcal surface protein A (PspA) vaccine against pneumococcal challenge by five different PspA clades in mice. Vaccine 32, 5607–5613 (2014).
De Chiara, M. et al. Genome sequencing of disease and carriage isolates of nontypeable Haemophilus influenzae identifies discrete population structure. Proc. Natl Acad. Sci. USA 111, 5439–5444 (2014).
Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372, 835–845 (2015).
Shi, T. et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390, 946–958 (2017).
Scheltema, N. M. et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob. Health 5, e984–e991 (2017).
Yu, X. et al. Antibody and local cytokine response to respiratory syncytial virus infection in community-dwelling older adults. mSphere 5, e00577–20 (2020).
Venkatesan, P. First RSV vaccine approvals. Lancet Microbe 4, e577 (2023).
Schepens, B. et al. Protection and mechanism of action of a novel human respiratory syncytial virus vaccine candidate based on the extracellular domain of small hydrophobic protein. EMBO Mol. Med. 6, 1436–1454 (2014).
Umemoto, S. et al. Cationic-nanogel nasal vaccine containing the ectodomain of RSV-small hydrophobic protein induces protective immunity in rodents. NPJ Vaccines 8, 106 (2023). This paper demonstrated the feasibility of developing a nasal vaccine that induces both humoral and cell-mediated immunity against RSV infection.
van Hoogevest, P. & Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 116, 1088–1107 (2014).
Sia, Z. R. et al. A liposome-displayed hemagglutinin vaccine platform protects mice and ferrets from heterologous influenza virus challenge. Proc. Natl Acad. Sci. USA 118, e2025759118 (2021).
Islam, N. & Ferro, V. Recent advances in chitosan-based nanoparticulate pulmonary drug delivery. Nanoscale 8, 14341–14358 (2016).
Sonaje, K. et al. Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: microscopic, ultrastructural, and computed-tomographic observations. Mol. Pharm. 9, 1271–1279 (2012).
Gong, X., Gao, Y., Shu, J., Zhang, C. & Zhao, K. Chitosan-based nanomaterial as immune adjuvant and delivery carrier for vaccines. Vaccines 10, 1906 (2022).
Xing, L. et al. Chemical modification of chitosan for efficient vaccine delivery. Molecules 23, 229 (2018).
Mills, K. H. et al. Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin a. Infect. Immun. 71, 726–732 (2003).
Atmar, R. L. et al. Norovirus vaccine against experimental human Norwalk Virus illness. N. Engl. J. Med. 365, 2178–2187 (2011).
El-Kamary, S. S. et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 202, 1649–1658 (2010).
Patel, D. R. et al. Intranasal SARS-CoV-2 RBD decorated nanoparticle vaccine enhances viral clearance in the Syrian hamster model. Microbiol. Spectr. 12, e0499822 (2024).
Wagstaffe, H. R. et al. Mucosal and systemic immune correlates of viral control after SARS-CoV-2 infection challenge in seronegative adults. Sci. Immunol. 9, eadj9285 (2024).
Park, H. S. et al. Intranasal immunization with avian paramyxovirus type 3 expressing SARS-CoV-2 spike protein protects hamsters against SARS-CoV-2. NPJ Vaccines 7, 72 (2022).
Le Nouen, C. et al. Intranasal pediatric parainfluenza virus-vectored SARS-CoV-2 vaccine is protective in monkeys. Cell 185, 4811–4825.e4817 (2022).
Liu, X. et al. A single intranasal dose of a live-attenuated parainfluenza virus-vectored SARS-CoV-2 vaccine is protective in hamsters. Proc. Natl Acad. Sci. USA 118, e2109744118 (2021).
van Doremalen, N. et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 13, eabh0755 (2021).
Ying, B. et al. Mucosal vaccine-induced cross-reactive CD8+ T cells protect against SARS-CoV-2 XBB.1.5 respiratory tract infection. Nat. Immunol. 25, 537–551 (2024).
Madhavan, M. et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial. eBioMedicine 85, 104298 (2022).
Mudrick, H. E. et al. Comparison of replicating and nonreplicating vaccines against SARS-CoV-2. Sci. Adv. 8, eabm8563 (2022).
Ahi, Y. S., Bangari, D. S. & Mittal, S. K. Adenoviral vector immunity: its implications and circumvention strategies. Curr. Gene Ther. 11, 307–320 (2011).
Adler, J. M. et al. An intranasal live-attenuated SARS-CoV-2 vaccine limits virus transmission. Nat. Commun. 15, 995 (2024).
Mao, T. et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science 378, eabo2523 (2022). This paper presented a vaccine strategy of ‘prime and spike’, which uses the existing systemic immunity induced by primary injection vaccination (prime) to induce mucosal immune memory within the respiratory tract by using unadjuvanted intranasal spike boosters (spike) for protection against SARS-CoV-2 infection.
McMahan, K. et al. Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques. Nature 626, 385–391 (2024). This study showed that intratracheal booster vaccination with an Ad26-based bivalent SARS-CoV-2 vaccine in systemically primed non-human primates induced potent mucosal humoral and cellular immunity that provided near-complete protection against a SARS-CoV-2 BQ.1.1 challenge.
Gagne, M. et al. Mucosal adenovirus vaccine boosting elicits IgA and durably prevents XBB.1.16 infection in nonhuman primates. Nat. Immunol. 25, 1913–1927 (2024).
Chen, J. et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci. Bull. 67, 1372–1387 (2022).
Sun, W. et al. A Newcastle disease virus (NDV) expressing a membrane-anchored spike as a cost-effective inactivated SARS-CoV-2 vaccine. Vaccines 8, 771 (2020).
Logunov, D. Y. et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 396, 887–897 (2020).
Beavis, A. C. et al. Efficacy of parainfluenza virus 5 (PIV5)-vectored intranasal COVID-19 vaccine as a single dose primer and booster against SARS-CoV-2 variants. J. Virol. 99, e01989-24 (2025).
An, D. et al. Protection of K18-hACE2 mice and ferrets against SARS-CoV-2 challenge by a single-dose mucosal immunization with a parainfluenza virus 5-based COVID-19 vaccine. Sci. Adv. 7, eabi5246 (2021).
Tioni, M. F. et al. Mucosal administration of a live attenuated recombinant COVID-19 vaccine protects nonhuman primates from SARS-CoV-2. NPJ Vaccines 7, 85 (2022).
Wang, Y. et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc. Natl Acad. Sci. USA 118, e2102775118 (2021).
O’Neill, A. et al. Mucosal SARS-CoV-2 vaccination of rodents elicits superior systemic T central memory function and cross-neutralising antibodies against variants of concern. eBioMedicine 99, 104924 (2024).
Yunis, J., Short, K. R. & Yu, D. Severe respiratory viral infections: T-cell functions diverging from immunity to inflammation. Trends Microbiol. 31, 644–656 (2023).
Schlom, J. & Donahue, R. N. The importance of cellular immunity in the development of vaccines and therapeutics for COVID-19. J. Infect. Dis. 222, 1435–1438 (2020).
Kalimuddin, S. et al. Vaccine-induced T cell responses control Orthoflavivirus challenge infection without neutralizing antibodies in humans. Nat. Microbiol. 10, 374–387 (2025).
Gonzalez Delgado, C. A. Adaptive phase I / II clinical trial, randomized, of parallel groups, to evaluate the safety and immunogenicity in adults of two vaccine candidates, based on recombinant RBD subunits for the prevention of COVID-19 in regimens that use the nasal route of administration (RPCEC, accessed 2 March 2024); https://rpcec.sld.cu/en/trials/RPCEC00000345-En.
Lam, J. H. et al. Next-generation intranasal Covid-19 vaccine: a polymersome-based protein subunit formulation that provides robust protection against multiple variants of concern and early reduction in viral load of the upper airway in the golden Syrian hamster model. Preprint at bioRxiv https://doi.org/10.1101/2022.02.12.480188 (2022).
Lam, J. H. et al. Polymersomes as stable nanocarriers for a highly immunogenic and durable SARS-CoV-2 spike protein subunit vaccine. ACS Nano 15, 15754–15770 (2021).
Li, J. X. et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir. Med. 10, 739–748 (2022).
Zhou, R. et al. Nasal prevention of SARS-CoV-2 infection by intranasal influenza-based boost vaccination in mouse models. eBioMedicine 75, 103762 (2022).
Hassan, A. O. et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183, 169–184.e113 (2020).
Bricker, T. L. et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 36, 109400 (2021).
Banihashemi, S. R. et al. Safety and efficacy of combined intramuscular/intranasal RAZI-COV PARS vaccine candidate against SARS-CoV-2: a preclinical study in several animal models. Front. Immunol. 13, 836745 (2022).
Logunov, D. Y. et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397, 671–681 (2021).
Liu, T., Liang, Y. & Huang, L. Development and delivery systems of mRNA vaccines. Front. Bioeng. Biotechnol. 9, 718753 (2021).
Yang, L., Tang, L., Zhang, M. & Liu, C. Recent advances in the molecular design and delivery technology of mRNA for vaccination against infectious diseases. Front. Immunol. 13, 896958 (2022).
Li, M. et al. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J. Control. Release 228, 9–19 (2016).
Shah, P., Lalan, M. & Barve, K. Intranasal delivery: An attractive route for the administration of nucleic acid based therapeutics for CNS disorders. Front. Pharmacol. 13, 974666 (2022).
Dhaliwal, H. K., Fan, Y., Kim, J. & Amiji, M. M. Intranasal delivery and transfection of mRNA therapeutics in the brain using cationic liposomes. Mol. Pharm. 17, 1996–2005 (2020).
Suberi, A. et al. Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination. Sci. Transl. Med. 15, eabq0603 (2023).
Baldeon Vaca, G. et al. Intranasal mRNA-LNP vaccination protects hamsters from SARS-CoV-2 infection. Sci. Adv. 9, eadh1655 (2023).
Waltz, E. How nasal-spray vaccines could change the pandemic. Nature 609, 240–242 (2022).
Topol, E. J. & Iwasaki, A. Operation Nasal Vaccine—lightning speed to counter COVID-19. Sci. Immunol. 7, eadd9947 (2022).
Lavelle, E. C. & Ward, R. W. Mucosal vaccines—fortifying the frontiers. Nat. Rev. Immunol. 22, 236–250 (2022).
US Food and Drug Administration. FluMist Quadrivalent. FDA https://www.fda.gov/vaccines-blood-biologics/vaccines/flumist-quadrivalent (2023).
Carter, N. J. & Curran, M. P. Live attenuated influenza vaccine (FluMist; Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs 71, 1591–1622 (2011).
Jeyanathan, M. et al. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 20, 615–632 (2020).
Vignuzzi, M., Wendt, E. & Andino, R. Engineering attenuated virus vaccines by controlling replication fidelity. Nat. Med. 14, 154–161 (2008).
Aoshi, T. Modes of action for mucosal vaccine adjuvants. Viral Immunol. 30, 463–470 (2017).
US Centers for Disease Control and Prevention. Adjuvants and Vaccines. CDC https://www.cdc.gov/vaccine-safety/about/adjuvants.html (2024).
Zhao, T. et al. Vaccine adjuvants: mechanisms and platforms. Signal Transduct. Target Ther. 8, 283 (2023).
Wrobel, B. B. & Leopold, D. A. Olfactory and sensory attributes of the nose. Otolaryngol. Clin. North Am. 38, 1163–1170 (2005).
Alvites, R. D. et al. The nasal cavity of the rat and mouse–source of mesenchymal stem cells for treatment of peripheral nerve injury. Anat. Rec. 301, 1678–1689 (2018).
Ramvikas, M., Arumugam, M., Chakrabarti, S. R. & Jaganathan, K. S. in Micro and Nanotechnology in Vaccine Development (eds Skwarczynski, M. & Toth, I.) 279–301 (Elsevier, 2017).
Verma, A. K., Zheng, J., Meyerholz, D. K. & Perlman, S. SARS-CoV-2 infection of sustentacular cells disrupts olfactory signaling pathways. JCI Insight 7, e160277 (2022).
Mutsch, M. et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 350, 896–903 (2004).
Miller, E. et al. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. Brit. Med. J. 346, f794 (2013).
Nohynek, H. et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS ONE 7, e33536 (2012).
Stowe, J. et al. Risk of narcolepsy after AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine in adults: a case-coverage study in England. Sleep 39, 1051–1057 (2016).
Dauvilliers, Y. et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain 136, 2486–2496 (2013).
Fukuyama, Y. et al. Nasal administration of cholera toxin as a mucosal adjuvant damages the olfactory system in mice. PLoS ONE 10, e0139368 (2015).