Reagents
Chemicals used for cell death assays were dissolved in DMSO. 17β-oestradiol, testosterone and 2-hydroxyoestradiol were dissolved in ethanol. A list of sources of all chemicals is provided in the Supplementary Information.
Cell lines and cell culture
A list of all cell lines used in this study is provided in the Supplementary Information. Human HT1080, HT29, HeLa and mouse NIH-3T3 cell lines were purchased from the American Type Culture Collection, while CD10-135 cells were provided by collaborators. No further validation was initiated. All cell lines underwent regular testing for mycoplasma infection, and all cell lines were grown in a humidified 5% CO2 atmosphere at 37 °C. HT1080, HT29, HeLa and NIH-3T3 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher Scientific) supplemented with 10% (v/v) FBS (Thermo Fisher Scientific, 41966029), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Thermo Fisher Scientific, 15140122). CD10-135 cells were cultured in DMEM F-12 Nutrient Mixture with Glutamax (DMEM/F12 Glutamax, Thermo Fisher Scientific, 10565018) supplemented with 10% (v/v) FBS (Thermo Fisher Scientific, 41966029), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Thermo Fisher Scientific, 15140122).
CRISPR–Cas9-mediated gene knockout
Sequences of all guides are provided in the Supplementary Information. The plasmid pSpCas9(BB)-2A-Blast (Addgene, 118055), which contains a blasticidin-resistance gene, was used to insert guide RNAs (gRNAs) targeting various human genes. The plasmid was linearized using BbsI-HF (NEB, R3539L), and gRNAs targeting the human genes CBS, CTH, AIFM2, ESR1 and FAR1 were inserted (the sequences are provided in the Supplementary Information). NEB 5-alpha competent Escherichia coli (high efficiency, NEB, C2987H) were transformed with plasmids according to the manufacturer’s instructions and colonies were grown overnight over LB agar plates (BD Biosciences, 244520) supplemented with 0.1 mg ml−1 ampicillin (Roth, 200-708-1) at 37 °C. Single colonies were picked for plasmid propagation and isolation (Macherey-Nagel, 740410.50). To verify plasmid integrity, the isolated plasmids were digested with SacI-HF (NEB, R3156L) and resolved on 1% agarose gel. Transfection was performed using the Neon NxT electroporation system (Thermo Fisher Scientific, NEON1SK), with the electroporation conditions set at 3 pulses of 10 ms at 1,650 V.
HT29 cells were electroporated with plasmids carrying gRNAs targeting CBS, CTH, AIFM2 and ESR1. Selection was initiated 24 h after transfection using 40 µg ml−1 blasticidin (Invivogen, ant-bl-05) and maintained for 2 weeks. Similarly, HT1080 and CD10 cells were electroporated with plasmids containing gRNAs targeting FAR1, followed by selection with 20 µg ml−1 and 40 µg ml−1 blasticidin, respectively, starting 24 h after transfection for 2 weeks. Knockout efficiency was verified by western blot in polyclonal cell populations. The guide sequences are provided in the Supplementary Information.
Plating and treatment of cells
Detachment of HT1080, HT29, HeLa, NIH-3T3 and CD10 cells was performed using trypsin-EDTA (Gibco, 25200056). Cells were then washed twice and seeded in six-well plates (Sarstedt, 83.3920). All cells were seeded at 1 × 105 cells per well in six-well plates. Before the treatment, the medium was changed. Experiments were performed in a total volume of 1 ml.
Cell death assays
Ferroptosis was induced using established FINs: type I FIN, erastin (Sigma-Aldrich); type II FIN, RSL3 (Selleckchem); type III FIN, FIN56 (Sigma-Aldrich); and type IV FIN, FINO2 (Cayman Chemical). Necrosis was additionally induced as previously described by the thioredoxin reductase inhibitor FTC30. Unless otherwise indicated, we used 5 μM erastin, 1.13 μM RSL3, 10 μM FIN56, 10 μM FINO2 and 10 μM FTC. After the indicated timepoints, cells were collected and prepared for flow cytometry or western blotting.
Flow cytometry
Cells were collected and the pellets were washed twice in PBS and stained with 5 μl of 7-AAD (BD Biosciences) and 5 μl of annexin-V–FITC (BD Biosciences) added to 100 μl annexin-V binding buffer solution (BD Biosciences). After 15 min, cells were recorded either on the Fortessa LSRII system with the FACS Diva 6.1.1 software (BD Biosciences), or on the Symphony A3 system with the FAVS Diva v9.0 software (BD Biosciences) and subsequently analysed with the FlowJo v.10 software (Tree Star). The flow cytometry procedure was supported by the Flow Cytometry Core Facility of the CMCB Technology Platform at Technical University of Dresden (TU Dresden) and the FACS Facility of the Institute for Physiological Chemistry (TU Dresden).
Western blotting
Cells were lysed in ice-cold 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 5 mM EDTA supplemented with PhosSTOP (Merck), cOmplete (Merck) and 1 mM phenylmethylsulfonyl fluoride for 30 min on ice. Insoluble material was removed by centrifugation (14,000g, 30 min, 4 °C). The protein concentration was determined using a commercial BCA assay kit according to the manufacturer’s instructions (Thermo Fisher Scientific). Equal amounts of protein (typically 25 μg per lane) were resolved on a 4–15% gradient SDS–PAGE gel and transferred to a PVDF membrane (Bio-Rad). After blocking for 1 h at room temperature, incubation with primary antibody was performed at 4 °C overnight. Primary antibodies ACSL4 (Abcam, ab155282), GPX4 (Abcam, ab125066), CBS (Thermo Fisher Scientific, MA5-17273), CSE (Proteintech, 60234-1-Ig), POR (Abcam, ab180597), ETHE1 (GeneTex, GTX115707) and SQR (Abcam, ab71978) were diluted 1:1,000 in 5% BSA (Serva, 9048-46-8). Primary antibodies PRX (Abcam, ab184868), AGPS (Invitrogen, A115277), FAR1 (Novus Biological, A107209), FSP1 (provided by M. Conrad, 14D7, or Santa Cruz Biotechnology, sc-377120) and β-actin (Cell Signaling, 3700S) were diluted 1:1,000 in low-fat milk (Roth, 68514-61-4). Secondary antibodies, anti-mouse HRP-linked antibody (Cell Signaling, 7076S) and anti-rabbit HRP-linked antibody (Cell Signaling, 7074S), were applied at concentrations of 1:5,000. Proteins were then visualized by enhanced chemiluminescence (ECL, Amersham Biosciences).
Western blot analysis of renal tubules
For western blot analysis of kidney tubules, freshly isolated tubules are transferred into a 2 ml reaction tube. Depending on the pellet size, an approximate volume of 7 to 30 μl of Roti Load 1 (Roth, K929.1) is added, mixed thoroughly and subsequently snap-frozen in liquid nitrogen. The samples were then either stored at −80 °C or used directly for the western blot analysis. It is essential to assess the protein loading equivalency, as a classical Bradford assay cannot be conducted. Between 3 and 7 μl of tubules, contingent on the quantity, was loaded onto the gel. Next, Ponceau staining was performed to evaluate the uniformity of protein loading. If the staining results were consistent, the protocol proceeded as previously outlined for cell samples.
Isolation of primary mouse renal tubules
Primary mouse renal tubules were isolated strictly following a recently published protocol43. In detail, mouse kidneys were removed, washed with PBS, decapsualized and sliced in four to five slices. Kidney slices of each kidney were transferred into a 2 ml reaction tube containing 2 mg ml−1 collagenase type II in incubation solution (48 μg ml−1 trypsin inhibitor, 25 μg ml−1 DNase I, 140 mM NaCl, 0.4 mM KH2PO4, 1.6 mM K2HPO4·3H2O, 1 mM MgSO4·7H2O, 10 mM CH3COONa·3H2O, 1 mM a-ketoglutarate and 1.3 mM Ca-gluconate) and digested for 5 min at 37 °C, 850 rpm. Owing to the presence of damaged tubules, the first resulting supernatant was discarded and 1 ml of incubation solution was added to the kidney slices and digested for 5 min at 37 °C, 850 rpm. The supernatant was collected and transferred in a 2 ml reaction tube containing 1 ml ice-cold sorting solution (0.5 mg ml−1 bovine albumin in incubation solution). The reaction tubes were left on ice for the tubules to precipitate. The supernatant was removed and the tubules were washed twice with ice-cold incubation solution. Once the tubules precipitated, the supernatant was removed and ice-cold sorting solution was added (the volume was adjusted depending on the number of samples needed for the experiment). Tubules were distributed in a 24-well plate containing DMEM F-12 nutrient mixture without glycine and phenol red (DMEM/F12, custom-made medium provided by Cell Culture Technologies), supplemented with 0.01 mg ml−1 recombinant human insulin, 5.5 μg ml−1 human transferrin, 0.005 μg ml−1 sodium selenite (Na2SeO3) and 470 μg ml−1 linoleic acid (ITS+1, Sigma-Aldrich, I2521), 50 nM hydrocortisone, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Thermo Fisher Scientific).
Investigation of pig kidney tissue
Six-month-old INSC94Y (n = 1, male)44 and non-transgenic littermates (n = 2 (male) and n = 2 (female)) as well as one 2.5-year-old male INSC94Y transgenic boar and a 3.5-year-old non-transgenic boar were euthanized. The kidneys were immediately explanted and further processed for tubule isolation. All of the experiments were performed according to the German Animal Welfare Act with permission from the responsible authority (Government of Upper Bavaria, ROB-55.2-2532.Vet-02-19-195), according to the ARRIVE guidelines and Directive 2010/63/EU. Investigators were strictly blinded to experimental groups during data acquisition and analysis.
Primary porcine renal tubules were isolated using an identical procedure along with the corresponding solutions and media to those described for mouse tubules. Owing to the larger size of the porcine kidney, multiple 2 ml reaction tubes were used during the isolation process.
Human renal tubules
Human renal tissue for the isolation of renal tubules was obtained from fresh tumour nephrectomy specimens at the University Hospital Dresden. Informed consent was obtained from all patients; ethical approval was granted through the uro-oncological biobanking agreement. All aspects of the declaration of Helsinki were met. After open nephrectomy, tissue was immediately obtained from a non-tumour-infiltrated area. Appropriate sections of the kidney medulla, lacking tumour tissue, were excised from the kidneys by medical professionals. The sections were stored in 50 ml reaction tubes in PBS until they could be sliced into thin sections in the laboratory, following the same protocol used for mouse and porcine tubules. Owing to the size of the sections, up to ten 2 ml tubes were used. The remainder of the protocol was consistent with that used for the isolation of porcine and murine tubules; however, the incubation times for digestion and precipitation were extended to 10 min due to the higher density of human tissue composition, which complicates the sectioning process. Approval for use of nephrectomy samples was granted by the ethics commission of the TU Dresden (EK194092004). Informed consent for use of extant tissue for research purposes was obtained before biopsy. Refusal did not affect clinical care. Investigators were strictly blinded to experimental groups during data acquisition and analysis.
Human renal biopsies
For the set of human renal biopsies used in this study, we identified samples of patients who underwent renal indication biopsy for mild-to-medium chronic kidney disease of uncertain aetiology at the University Hospital Dresden from 2022 to 2025 that demonstrated minimal chronic tubular injury due to IgA nephritis. Importantly, no other significant comorbidities were present in these patients except for well-controlled hypertension in some cases. The mean ages of the patients were as follows: male (30.25 years), premenopausal female (32.0 years) and postmenopausal female (62.0 years). Fresh biopsy materials were fixed in 4% normal buffered formalin for at least 24 h before embedding in paraffine. After deparaffinization with xylene and rehydration with graded ethanols, unspecific binding was blocked with 3% BSA in PBS and background sniper (50-823-84, Biocare Medical). Subsequently, the primary antibody was incubated at a concentration of 1:5,000 for AGPS (ab236621, Abcam) followed by anti-rabbit secondary antibody (7074S, Cell Signaling). Bound antibody was visualized with a standard polymer horseradish peroxidase system and counterstained with haematoxylin. Stained sections were analysed using the Axio Imager microscope (Zeiss) or Zeiss Observer Z.1 at ×100, ×200 and ×400 magnification. Micrographs were digitalized using an AxioCam MRm Rev. 3 FireWire camera and AxioVision v.4.5 software (Zeiss), or using an AxioCam MRc and Zen 2012 Software (Zeiss), respectively. Semiquantative scoring of the immunohistochemistry staining intensity (ranging from 0 to 3) in the brush border compartment of proximal tubules was performed by an experienced nephropathologist in a strictly double-blinded manner. Approval for use of human renal biopsies was granted by the ethics commission of the TU Dresden (EK 148052012 and BOK-EK-431102023). Informed consent for use of extant tissue for research purposes was obtained before biopsy. Refusal did not affect clinical care. Investigators were strictly blinded to experimental groups during data acquisition and analysis.
Human renal gene expression data
We explored the MetMap500 database through the DepMap portal45 (https://depmap.org/metmap/vis-app/index.html; last accessed 1 April 2025) and identified 11 ESR1+ as well as 12 ESR1− breast cancer cell lines. As all ESR-negative cell lines were also negative for HER2, only ESR1+HER2− and ESR1−HER2− cell lines (7 versus 12) were compared directly. To this end, batch-corrected expression data (Public 24Q4, https://doi.org/10.25452/figshare.plus.27993248.v1) were plotted through the DepMap data explorer 2.0 tool of the Broad Institute37. These data were also visualized as a correlation heat map using the same tool.
To compare gene expression data in healthy individuals and patients with AKI, we used an approach published previously of merging scRNA data from KPMP and the Human Kidney Single Cell Transcriptome36. These data were analysed using a tool publicly provided by the Suztak Laboratory (https://susztaklab.com/hk_genemap_kpmp/scRNA; last accessed 2 April 2025).
Assessment of tubular necrosis and treatments of murine renal tubules
All experiments included a negative control to assess LDH release at 0 h. No more than 10% LDH release in these negative controls was tolerated as a quality control. Freshly isolated mouse renal tubules were placed in 24-well plates in DMEM/F12 nutrient mixture without glycine and phenol red (DMEM/F12, custom-made medium provided by Cell Culture Technologies), supplemented with 0.01 mg ml−1 recombinant human insulin, 5.5 μg ml−1 human transferrin, 0.005 μg ml−1 Na2SeO3 and 470 μg ml−1 linoleic acid (ITS + 1, Sigma-Aldrich, I2521), 50 nM hydrocortisone, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin (Thermo Fisher Scientific). After the indicated times, the medium of each well was collected and tubules were prepared for an LDH-release assay (see the ‘LDH-release assay’ section for further details).
In the case of treatment of tubules, the medium or each well of a 24-well plate was incubated with the compounds of interest. Tubules were added to each well and the samples were prepared for the LDH-release assay.
LDH-release assay
The LDH release of cells or of freshly isolated kidney tubules was measured according to manufacturers’ instructions at the indicated timepoints. In brief, an aliquot of the supernatant was taken to assess the experimental LDH values. Subsequently, lysis solution was added for 45 min to induce maximal LDH release before another aliquot of the supernatant was taken. The supernatants were then incubated with CytoTox 96 Reagent for 15 min protected from the light at room temperature before adding stop solution.
Absorbance was measured at 490 nm and calculated as \(100\times \frac{{\rm{experimental}}\;{\rm{LDH}}\;{\rm{release}}}{\text{max LDH release}}\).
Time-lapse imaging
Videos of freshly isolated mouse tubules stained with 50 nM SYTOX Green nucleic acid stain (Life Technologies) in the presence or absence of 150 nM Biotracker 609 Red Ca2+ AM dye (Merck Millipore, 5.04297.0001) or 200 nM MitoTracker Red FM (Invitrogen, M22425) were obtained using an oil-immersion ×63/0.3 EC Plan Neofluar objective. For these experiments, high-quality plastic-bottom slides (Ibidi 15 μ-slide 8-well, 80826) were used. The comparison of male and female mouse tubules was performed using a ×2.5/0.3 EC Plan Neofluar objective, while the tubules were plated in a custom-made 3D chamber. An Axiovert 200M or a Zeiss Observer Z.1, both equipped with a large incubation chamber (37 °C), 5% CO2 and humidity control were used for all of the live imaging experiments. Transmitted light and fluorescence images (GFP BP filter cube, RFP double filter cube) were acquired using an Orca flash 4.0 camera (Axiovert) or an Axiocam 506 colour (Zeiss Observer Z.1). The live imaging procedure was supported by the Light Microscopy Facility, a Core Facility of the CMCB Technology Platform at Technical University of Dresden (TU Dresden), and the CFCI Core Facility Cellular Imaging (TU Dresden).
Quantification of SYTOX positivity in freshly isolated renal tubules
Isolated renal tubules from male or female mice were incubated in a single 3D-printed well separated by a glass slide, stained with SYTOX green nucleic acid stain. Transmitted light and fluorescence time-lapse images (GFP BP filter cube) were acquired (described in more detail in the ‘Time-lapse imaging’ section). Every 30 min, the images were assessed for the number of tubules exhibiting more than 90% of SYTOX green positivity. For the quantification of cell death propagation, tubules with less than 90% SYTOX positivity were not counted. Debris was not included in the analysis. The total numbers of male and female tubules were visually counted. Data are presented as the percentage of tubules with equal or more than 90% of SYTOX positivity over time.
Electron microscopy
Mouse tubules were isolated according to the above-mentioned protocol, fixed in 4% buffered paraformaldehyde and then fixed in glutaraldehyde and underwent 1 h of post-fixation/contrasting with osmium tetroxide. The samples were then embedded in Epon resin through graded ethanols and propylene oxide. Blocks were polymerized at 80 °C overnight. Semi-thin sections were stained with methylene blue and azure blue. Thin sections were stained with lead citrate and uranyl acetate. Transmission electron microscopy was performed on the Zeiss Electron Microscope EM 906 (Oberkochem). A Wide-Angle Dual-Speed 2K CCD-Camera TRS 465/14 (Tröndle Restlichtverstärkersysteme) was used for image acquisition in combination with Image SP (Tröndle Restlichtverstärkersysteme) as software. Photoshop 2024 for Macintosh (Adobe) was used for final image preparation.
Inhibited egg phosphatidylcholine liposome co-autoxidations (FENIX 1.0)
RTA only
Egg phosphatidylcholine liposomes (1.02 mM) (prepared as previously described46) STY-BODIPY (1.02 μM) and di-tert-undecyl hyponitrite (DTUN) (0.203 mM) in chelex-treated phosphate-buffered saline (cPBS) (12 mM phosphate, 150 mM NaCl, pH 7.4) were added to the wells of a Nunc black polypropylene round-bottomed 96-well microplate (295 μl). Using a 1–10 μl multichannel pipette, 5 μl of inhibitor solution in DMSO or vehicle only was then added to afford a final volume of 300 μl (final concentration of 1 mM egg-PC, 1 μM STY-BODIPY, 0.2 mM DTUN and inhibitor varying between 2 and 16 μM). The reaction mixtures were manually mixed using a 100 to 300 μl multichannel pipette (set to 250 μl) and the microplate was inserted into a BioTek H1 Synergy microplate reader equilibrated to 37 °C and vigorously shaken for 1 min followed by a 3.5-min delay. Fluorescence was then recorded (λex = 488 nm; λem = 518 nm; gain = 60) every minute for 6 h. For co-extruded samples, egg phosphatidylcholine liposomes (final concentration 1.02 mM) (prepared as described above) and inhibitor solution in DMSO (final concentration varied between 2.05 and 16.37 µM) were added to cPBS, vortexed and re-extruded through a 100 nm polycarbonate membrane 15 times. This co-extruded mixture (782 µl) was subsequently diluted with STY-BODIPY (80 µM, 10 µl) and DTUN (20 mM, 8 µl) to yield a final volume of 800 µl (final concentration of 1 mM egg-PC, 1 µM STY-BODIPY, 0.2 mM DTUN, inhibitor varying between 2 and 16 µM in microplate well). The final solution was vortexed for 5 s and 300 µl was plated per well (two wells plated per condition) and the fluorescence was recorded on the BioTek H1 Synergy microplate as described above.
RTA + reductant
Egg phosphatidylcholine liposomes (1.02 mM) (prepared as previously described46), STY-BODIPY (1.02 μM), inhibitor (4.07 µM) and DTUN (0.203 mM) in cPBS (12 mM phosphate, 150 mM NaCl, pH 7.4) were added to the wells of a Nunc black polypropylene round-bottomed 96-well microplate (295 μl). Using a 1–10 μl multichannel pipette, 5 μl of reductant solution in cPBS or vehicle only was then added to afford a final volume of 300 μl (final concentration of 1 mM egg-PC, 1 μM STY-BODIPY, 4 µM inhibitor, 0.2 mM DTUN and reductant concentration varies between 4 µM and 100 µM). The reaction mixtures were manually mixed using a 100–300 μl multichannel pipette (set to 250 μl) and the microplate was inserted into a BioTek H1 Synergy microplate reader equilibrated to 37 °C and vigorously shaken for 1 min followed by a 3.5 min delay. The fluorescence was then recorded (λex = 488 nm; λem = 518 nm; gain = 60) every minute for 15 h.
RTA + mFSP1
Egg phosphatidylcholine liposomes (1.03 mM) (prepared as previously described46), STY-BODIPY (1.03 μM), inhibitor (4.14 µM), mFSP1 (16.55 nM) and FAD (331 nM) in pH 7.4 TBS buffer were added to the wells of a Nunc black polypropylene round-bottomed 96-well microplate (290 μl). Using a 1–10 μl multichannel pipette, 5 μl of NADPH solution in TBS or vehicle only was then added followed by 5 µl of DTUN solution (12 mM) in ethanol to give a final volume of 300 μl (final concentration of 1 mM egg-PC, 1 μM STY-BODIPY, 4 µM inhibitor, 16 nM mFSP1, 320 nM FAD, 0.2 mM DTUN and NADPH varying between 4 and 64 μM). The reaction mixtures were manually mixed using a 100–300 μl multichannel pipette (set to 250 μl) and the microplate was inserted into the BioTek H1 Synergy microplate reader equilibrated to 37 °C and vigorously shaken for 1 min followed by a 3.5 min delay. Fluorescence was then recorded (λex = 488 nm; λem = 518 nm; gain = 60) every minute for 15 h. For co-extruded samples, egg-phosphatidylcholine liposomes (1.04 mM) and inhibitor in DMSO (4.15 µM) were added to TBS, vortexed and re-extruded through a 100 nm polycarbonate membrane 15 times. This co-extruded mixture (3594 µl) was then diluted with STY-BODIPY (1.74 mM, 2.14 µl), mFSP1 (48.9 µM, 1.22 µl) and FAD (0.5 mM, 2.38 µl) to achieve a final volume of 3,600 µl of bulk-lipid mixture. Then, 290 µl of bulk-lipid mixture was plated in a Nunc black polypropylene round-bottomed 96-well microplate, followed by 5 µl of NADPH solution in TBS or vehicle. Finally, 5 µl of DTUN solution in ethanol (12 mM) was added (the final concentrations in well were as follows: 1 mM egg-PC, 1 µM STY-BODIPY, 4 µM inhibitor, 16 nM mFSP1, 320 nM FAD, 4–64 µM NADPH and 0.2 mM DTUN). The reaction mixtures were manually mixed and the fluorescence was recorded on the BioTek H1 Synergy microplate as above.
Generation of 5,6,7,8-tetrahydronaphthalene-2,3-diol
To a pressure tube dried overnight at 150 °C in a drying oven was added under inert gas 2,3-dihydroxy-naphthalene (0.162 g, 1.01 mmol, 1.0 eq.), [Rh(cod)Cl]2 (75.0 mg, 0.015 mmol, 1.0 eq., 15 mol%) and polymethylhydrosiloxane (0.18 ml, 3.00 mmol, 3.0 eq.) in methanol (3 ml). The reaction mixture was stirred at room temperature for 2 days. The solvent was removed in a vacuum and the crude product was purified over silica using the running mixture of iso-hexane/ethyl acetate (2.5:1) with addition of 0.2 vol% triethylamine. The product was obtained as an off-white solid (118 mg, 72%). Then, 40 mg of the product was purified by high-performance LC (HPLC) using the running mixture of iso-hexane/ethyl acetate (5:1) and obtained as a colourless solid (8.6 mg, 21%). Rf(isoHex/EA: 3:1) = 0.43 (stained with anisaldehyde); 1H NMR (CDCl3, 300 MHz): δ (ppm) = 6.57 (s, 2H), 4.80 (s, 2H), 2.66–2.61 (m, 4H), 1.76–1.72 (m, 4H)47.
Measurement of sulfur-containing metabolites by ultra-performance LC–MS
Investigators were strictly blinded to experimental groups during data acquisition and analysis. In brief, the isolated tubules (see the ‘Isolation of primary mouse renal tubules’ section for the isolation protocol) were washed twice with cold NaCl (0.9%) solution. Subsequently, any excess liquid was carefully removed. To ensure cell lysis and alkylation of thiol and persulfide species, 200 µl of a 5 mM MBB (monobromobimane) solution in 50% methanol was added to the tubules. The samples were then incubated in the dark at room temperature for 20 min. After the incubation period, the tubules were snap-frozen in liquid nitrogen, stored at −80 °C and finally shipped to the designated location on dry ice for further analysis.
The cell suspension was centrifuged at 14,000g for 10 min, and 3 µl of supernatant was applied to an Accucore 150 Amide HILIC HPLC column (100 × 2.1 mm, 2.6 µm particle size) equipped with a guard cartridge (at 30 °C). Mobile phase A was 5 mM ammonium acetate in 5% acetonitrile (CH3CN); mobile phase B was 5 mM ammonium acetate in 95% CH3CN. The LC gradient program was: 98% B for 1 min, followed by a linear decrease to 40% B within 5 min, then maintain 40% B for 13 min, then return to 98% B in 1 min and finally 5 min at 98% B for column equilibration. The flow rate was 350 μl min−1. The eluent was directed to the electrospray ionization (ESI) source of the Q Exactive (QE) MS from 0.5 min to 19 min after sample injection. Each sample was run with the parallel reaction monitoring (PRM) method for monobromobimane alkylated metabolites. PRM method: scan type: PRM positive mode; runtime: 0.5–10 min. ddMS2 settings: resolution: 17,500; AGC target: 2 × 105; maximum injection time: 200 ms; loop count: 1; CE: 20, 50 and 80; isolation window: 1.2 m/z. For normalization among the samples, the protein pellets were dried on air and then dissolved in 200 µl 100 mM NaOH. The total protein content was determined by performing a BCA assay. PRM data were processed with Skyline48 (v.21.2.0.425) and normalized to total protein.
LC–MS/MS-based steroid hormone detection
Investigators were strictly blinded to experimental groups during data acquisition and analysis. Kidney tubules were isolated as described above and snap-frozen. To extract the lipophilic components, the tubules were homogenized in a 1.5 ml Eppendorf tube containing 250 µl of isopropanol and 10 µl of internal standard d-oestradiol (2,4,16,16-D4, 95-97%, DLM-2487, Cambridge Isotope Laboratories). Then, one-third volume of zirconium beads was added, and the sample was homogenized for 10 min at 4 °C and 300g in a TissueLyser II (Qiagen). After that, the sample was centrifuged for 10 min at 13,000g. The supernatant was transferred to a new Eppendorf tube, while the beads and pellet were reserved for protein quantification using the BCA Protein Quantification Kit (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific). The supernatant was incubated at −20 °C for 48 h or longer. After incubation, the sample was centrifuged again for 20 min at 4 °C and 13,000g, and the supernatant was transferred to another Eppendorf tube and desiccated in a vacuum desiccator.
For lipid extraction, MTBE extraction method was used. Then, 700 µl of a 10:3 mixture of MTBE and methanol (warmed to room temperature) was added to the dried sample. The sample was shaken for 1 h at 4 °C at 1,400 rpm. Then, 140 µl of water was added, and the sample was shaken again for 15 min at 4 °C at 1,400 rpm. The mixture was centrifuged for 15 min at 4 °C at 13,400 rpm. The upper organic phase was collected and transferred to a 1.5 ml Eppendorf tube, where it was evaporated.
For the derivatization with pyridine-3-sulfonyl chloride, 80 µl of sodium bicarbonate buffer (0.1 M, pH 10) and 80 µl of pyridine-3-sulfonyl chloride (2 mg ml−1 in acetone) were added to the dried extracts. The mixture was incubated at 60 °C for 15 min under generous shaking. After incubation, the mixture was cooled on ice for 10 min. The samples were centrifuged at 13,500 rpm at 4 °C for 10 min and the supernatant was collected for subsequent MS analysis.
Pyridine sulfonyl derivatives of oestradiol (E2), 2-hydroxyoestradiol (2OH-E2), 4-hydroxyoestradiol (4OH-E2) but also of the internal standard d4-oestradiol (d4-E2) were profiled by LC–tandem MS (LC–MS/MS) using an instrument set-up containing an ultra-performance LC system (Aquity I-class, Waters) coupled to a triple quadrupole linear ion-trap mass spectrometer (QTRAP 6500+, Sciex).
Chromatography separation was achieved using the Kinetex EVO C18 column (150 mm × 2.1 mm, 2.6 µm; Phenomenex) at 40 °C in conjunction with a gradient of aqueous mobile phase A (5 mM ammonium formate) and methanol as mobile phase B. Then, 5 μl of reconstituted samples, kept at 6 °C in the autosampler, were injected into the LC–MS/MS system at a flow rate of 0.350 ml min−1 with 57% mobile phase A. At 3.5 min, mobile phase B increased linearly to 46% until 4.0 min, followed by a decrease to 37% until 4.05 min, kept stable until 4.2 min, and then increased to 47.5% at 4.25 min. Next, mobile B was increased linearly to 85% until 9.5 min, then to 100% at 9.80 min. After a hold until 10.30 min, the gradient was returned back to the initial conditions at 10.80 min and was then maintained for another 3.20 min for column equilibration.
Derivatized oestrogens were analysed in multiple-reaction monitoring scan mode (MRM) using positive ESI including the ion source parameters curtain gas (40 psi), ESI voltage (5,500 V), source temperature (500 °C), gas 1 (70 psi) and gas 2 (50 psi). Compound-dependent source and fragmentation parameters were set to 100 V declustering potential, 10 V entrance potential, 45 V collision energy and 10 V cell exit potential.
For detection of E2 und d4-E2 (chromatographic retention time 9.3 min), respective pairs of quantifier and qualifier ions of 414.2–350.2 and 414.2–272.2, and 418.2–354.2 and 418.2–276.2 were used. Isobaric 4OH-E2 and 2OH-E2, baseline separated at retention times of 8.76 min and 8.96 min, respectively, were detected using pairs of 571.2–365.2 and 571.2–79.0.
Data acquisition was performed by Analyst 1.7 (Sciex). Data processing was done by using the Sciex OS-MQ software package. The data were analysed based on the peak area and normalized according to the internal standard and the total protein. For data presentation, the mean of values from the control group (E2 in males) was calculated and other values represented as factor of this mean.
Lipidomics analysis of murine renal tubules
Mouse renal tubules were freshly isolated according to the isolation of primary mouse renal tubules (see above; n = 6 for both sexes) and incubated in the presence of vehicle or 30 μM Fer-1 or 10 μM 2ΟΗ-Ε2. At 0 h and 6 h the supernatant was carefully removed, and tubules were subsequently snap-frozen in liquid nitrogen before storage at −80 °C before lipid extraction. Lipids were extracted according to the Folch method as previously described49. In brief, SPLASH LIPIDOMIX (Avanti Polar Lipids, 3 µl) and Cer/Sph Mixture I (Avanti Polar Lipids, 3 µl) internal lipid standards were added to each sample, incubated on ice for 15 min followed by the addition of ice-cold methanol (300 µl) and ice-cold chloroform (600 µl). The samples were vortexed and incubated at 4 °C for 1 h on a rotary shaker. Phase separation was induced by addition of ice-cold water (150 µl), followed by vortexing, incubation at 4 °C (10 min) and centrifugation (1,000g, 10 min, 4 °C). All extraction solvents contained 1 µg ml−1 butylated hydroxytoluene (BHT) to avoid oxidation. The organic phase was collected and dried in a vacuum concentrator.
For LC–MS analysis, lipids were resuspended in 50 µl of isopropanol and centrifuged, and 40 µl was transferred to glass vials. Lipids were separated by reversed-phase chromatography (Accucore C30 column; 150 mm × 2.1 mm 2.6 µM 150 Å, Thermo Fisher Scientific) using a Vanquish Horizon UHPLC system (Thermo Fisher Scientific) coupled on-line to the Orbitrap Exploris 240 mass spectrometer (Thermo Fisher Scientific) equipped with a HESI source. Lipids were separated at a flow rate of 0.3 ml min−1 (column temperature 50 °C) using the following gradient: 0–10 min, 30% to 80% B (curve 5); 10–27 min, 80% to 95% (curve 5); 27–31 min, 95% to 100% (curve 5); 31–37 min, isocratic 100% (curve 5); 37–42 min, re-equilibration at 30% B (curve 5). Eluent A consisted of acetonitrile:water (50:50, v/v, both ULC/MS-CC/SFC grade, Biosolve-Chemicals) and eluent B comprised 2-propanol:acetonitrile:water (85:10:5, v/v/v), both containing 5 mM ammonium formate (MS grade, Sigma-Aldrich) and 0.1% formic acid (ULC/MS-CC/SFC grade, Biosolve-Chemicals). Full MS settings were as follows: spray voltage, 3,500 V; sheath gas, 40 arb units; aux gas, 10 arb units; sweep gas, 1 arb unit; ion transfer tube, 300 °C; vaporizer temperature, 370 °C; EASY-IC run-start; default charge state, 1; resolution at m/z 200, 120,000; scan range, m/z 200–1,200; normalized AGC target, 100%; maximum injection time, auto; RF lens, 35%. Data-dependent acquisition was based on a cycle time (1.3 s) at a resolution of 30,000; isolation window, 1.2 m/z; normalized stepped collision energies, 17,27,37%; AGC target, 100%; maximum injection time, 54 ms.
Lipid identification was performed using Lipostar2. Features with isotopic pattern and MS/MS spectrum were matched against the LIPID MAPS database and selected based on automatic approval (3–4 stars). After manual approval based on an established LC–MS/MS lipid-identification strategy50, lipid identities and chromatographic peak areas were exported and used for further analysis. Peak areas were normalized according to lipid standard abundances of the SPLASH LIPIDOMIX and Cer/Sph Mixture I and protein content of the samples. Normalized peak areas were autoscaled using MetaboAnalyst 5.0. Lipids showing significant changes in abundance (analysis of variance (ANOVA), P < 0,01) were visualized through heat maps generated in Genesis v.1.8.1 (Bioinformatics TU-Graz). Investigators were strictly blinded to experimental groups during data acquisition and analysis.
Mice
Male and female mice (aged 8–12-week-old) were co-housed 2–5 mice per cage in individually ventilated cages in our facility at the Medizinisch-Theoretisches Zentrum (MTZ) at the Medical Faculty of the Technical University of Dresden (TU Dresden). All wild-type mice (C57BL/6N) were initially provided by Charles River at the age of 6–7 weeks. Aifm2−/− mice (B6.129-Aifm2tm1Marc/Ieg) were described from our laboratory previously21. Gsdmd–Mlkl-dKO mice (Mlkltm1.2Wsa × C57BL/6N-Gsdmdem4Fcw/J) were maintained in our facility and were described previously51. Gpx4fl/flROSA26-creERT2 mice were maintained in the Conrad laboratory in Munich as previously described20. CTH-deficient mice (Cthtm1lish) were described previously52 and were co-housed with wild-type littermates at groups of 2–5 mice at the Centre Hospitalier Universitaire Vaudois (University of Lausanne). Experiments on CTH-deficient mice were performed on-site in Lausanne as approved by local authorities. Esr1-deficient mice (B6N(Cg)-Esr1tm4.2Ksk/J)53 were purchased from Jackson Laboratories and bred from heterozygous male and female mice.
The genotype was validated by PCR of tail biopsies for all strains. If not otherwise specified, experiments were performed according to German animal protection laws and were approved by ethics committees and local authorities in Dresden (Landesdirektion Sachsen, Germany) or Munich (Germany) as described below.
Induction of Gpx4 knockout in mice
For survival and end-point studies, the tamoxifen-inducible conditional mouse strain Gpx4fl/flROSA26-creERT2 was used as previously described20. In brief, to induce Gpx4 deletion, tamoxifen was first dissolved in Miglyol at a concentration of 20 mg ml−1 (Tamoxifen, Sigma-Aldrich, T5648-1G; Miglyol, Caelo 3274-250mL), whereupon 100 μl was injected intraperitoneally on days 0 and 2. To detect potential gender-specific differences in survival time, the mice were monitored daily and euthanized by cervical dislocation after presenting with symptoms of acute kidney failure (humane end point). For the end-point study, mice were again injected with tamoxifen twice, and serum and kidneys were collected at day 10. All animals were bred and maintained under standard specific-pathogen free + individually ventilated cage conditions with food and water ab libitum and all studies were approved by the government of Upper Bavaria (Regierung von Oberbayern, Germany: ROB-55.2-2532.Vet_02-20-51).
Bilateral kidney IRI injury model
All male and female mice were strictly matched for weight, age and genetic background. Bilateral kidney IRI was performed as described in detail previously43. In essence, 30 min before anaesthesia, mice received a single intraperitoneal dose of a ferrostatin (Fer-1 (10 mg per kg at 200 µl), UAMC-2303 (200 µl 2.5 mM solution in 0.9% NaCl), or 2OH-oestradiol (10 mg per kg, 200 µl) or a corresponding vehicle control as indicated. Then, 15 min before surgery, all mice received 0.1 µg per g body weight buprenorphine-HCl for analgesia. Anaesthesia was induced by the application of 3 l min−1 of volatile isoflurane with pure oxygen in the induction chamber of a COMPAC5 (VetEquip) small animal anaesthesia unit. After achieving a sufficient level of narcosis, typically within 2 min, mice were placed in a supine position on a temperature-controlled self-regulated heating system calibrated to 38 °C and fixed with stripes at all extremities. Anaesthesia was reduced to a maintenance dose of 1.5 l min−1 isoflurane. Breathing characteristics and levels of analgesia were closely assessed visually. The abdomen was opened layer-by-layer to create a 2 cm wide opening. Blunt retractors (Fine Science Tools (FST)) were placed for convenient access. With the use of a surgical microscope (Carl Zeiss), sharp forceps were used to pinch retroperitoneal holes directly cranially and caudally in the renal pedicles. Using this access, a 100 g pressure micro serrefine (FST, 18055-03) was placed onto each pedicle to induce ischaemia. Time difference between the placement of both serrefines was recorded (typically <40 s, controlled in all cases to under 1:00 min), the gut was returned into the abdominal cavity and the opening was covered with the two gauze pieces. Then, 1 min before ending of target ischaemia time, the renal pedicles were visualized again and clamps removed exactly at the indicated times (1 s tolerance). The parietal peritoneum and the cutis, respectively, were closed separately by continuous seams using a 6-0 monocryl thread (Ethicon). Isoflurane application was stopped immediately thereafter and 1 ml of prewarmed PBS was administered intraperitoneally to compensate for any possible dehydration during surgery and to control for potential leakiness of the seams. The mice were divided into pairs of two and put back into the cages. 0.1 µg per g buprenorphine-HCl was administered every 8 h for analgesia. After a 48 h observation period, blood was collected by retroorbital puncture and the mice were euthanized by neck dislocation. The right kidney was removed to be fixed for 24 h in 4% normal buffered formalin and transferred to 70% ethanol for storage at room temperature. The left kidney was removed and shock frozen in liquid nitrogen before transfer to −80 °C for storage.
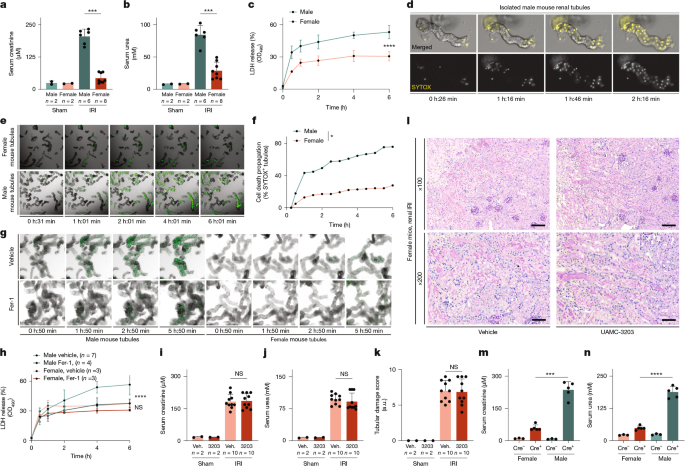
In this study, we applied different doses of ischaemia. For male mice, we defined 36 min as a hard ischaemia dose (Figs. 1a,b and 2j–m and Extended Data Fig. 5g,h). For female mice, three different doses were used from a titration experiment (Extended Data Fig. 1f–i) defining a medium ischaemia dose of 36 min (Figs. 2k–j,n–q and 3p–r and Extended Data Figs. 1b–e, 5i and 8c). A light ischaemia was defined as 30 min, whereas a hard ischaemia was set at 45 min (Figs. 1i–l and 4c–f and Extended Data Figs. 1a and 9a–d). Inhibitors and vehicle solution were freshly prepared and put on ice until use in a blinded manner. UAMC-3203 was applied intraperitoneally 15 min before surgery in 2.5 mM with 0.9% NaCl in a final volume of 200 µl, Fer-1 was applied intraperitoneally 15 min before surgery (5 mg per kg) in a 200 µl final volume and 2OH-E2 (10 mg per kg) in a 200 µl final volume.
To test the effect of ferrostatins in different doses of ischaemia, either vehicle or UAMC-3203 was applied 15 min before surgery as described above to 10-week-old C57BL/6N female wild-type mice. Surgery was performed as described above with ischaemia times escalating from 30 min to 45 min. After 48 h, blood was taken and the kidneys were removed and processed as described above (Supplementary Fig. 2j–l).
All IRI experiments were approved by the government of Saxony (Landesdirektion Sachsen, Germany; TVV 07/2021 and TVV 38/2024).
Ovariectomy
Ovariectomy was performed using an abdominal approach. At 15 min before surgery, all mice received 0.1 µg per g body weight buprenorphine-HCl for analgesia. Anaesthesia was induced by the application of volatile isoflurane with pure oxygen in the induction chamber of the COMPAC5 (VetEquip) small animal anaesthesia unit. After achieving a sufficient level of narcosis, typically within 2 min, mice were placed in a supine position on a temperature-controlled self-regulated heating system calibrated to 38 °C and fixed with stripes at all extremities. Surgical access to the abdominal cavity was created in the lower abdomen and the right ovary was visualized first. After ligation of the blood supply, the ovary was removed; the same procedure was applied to the left ovary. Next, the abdomen was closed layer by layer and the mice were set back into the individually ventilated cage in pairs. Then, 7 days later, IRI surgery was performed as described above partially using the previous abdominal access. Ovariectomies were approved by the government of Saxony (Landesdirektion Sachsen, Germany; TVV 38/2024).
Histology
Organs were dissected as indicated in each experiment and put in 4% (v/v) neutral-buffered formaldehyde, fixated for 24 h and then transferred to 70% ethanol for storage. In general, the kidneys were dehydrated in a graded ethanol series and xylene, and finally embedded in paraffin. Paraffin sections (3–5 μm) were stained with periodic acid–Schiff (PAS) reagent, according to a standard routine protocol. The stained sections were analysed using the Axio Imager microscope (Zeiss) or Zeiss Observer Z.1 at ×100, ×200 and ×400 magnification. Micrographs were digitalized using an AxioCam MRm Rev. 3 FireWire camera and AxioVision v.4.5 software (Zeiss), or using an AxioCam MRc and Zen 2012 Software (Zeiss), respectively. Organ damage was quantified by two experienced pathologists in a double-blinded manner on a scale ranging from 0 (unaffected tissue) to 10 (most severe organ damage). For the scoring system, tissues were stained with PAS, and the degree of morphological involvement in renal failure was determined using light microscopy. The following parameters were chosen as indicative of morphological damage to the kidney after IRI: brush border loss, red blood cell extravasation, tubule dilatation, tubule degeneration, tubule necrosis and tubular cast formation. These parameters were evaluated on a scale of 0–10, which ranged from not present (0), mild (1–4), moderate (5 or 6), severe (7 or 8), to very severe (9 or 10). Each parameter was determined on at least five different animals.
Software for illustrations
Illustrations were created using Affinity Designer 2.6, Affinity Photo 2.6 (Serif), Prism v.10.5.0 and Adobe Illustrator v.26.0.2.
Statistical analysis
Statistical analyses were performed using Prism 10 (GraphPad). For comparisons of single groups, a two-tailed parametric t-test with Welch’s correction (Figs. 1i–k,m,n, 2g,h,k–m,o–q, 3h,p–r and 4d–f,i and Extended Data Figs. 1b–d, 2b,c, 5f, 8a and 9a–c,k) or a nonparametric Mann–Whitney t-test (Fig. 1a,b) was used. For multiple or repeated comparisons, one-way ANOVA (Figs. 2b and 5i,s, Extended Data Figs. 3a–m, 4b–g, 5a,b, 6b,c, 7h,i and 9m and Supplementary Figs. 3a–m and 4b–d) or two-way ANOVA was used (Figs. 1c,h, 2i, 3i,o, 4a and 5g,j, Extended Data Figs. 7c, 8b and 9n and Supplementary Fig. 2d). To test the null hypothesis in the survival experiments, we plotted the animals in a Kaplan–Meier curve and used the log-rank test for statistics (Extended Data Fig. 2d). For dose–response curves, a four-parameter variable slope nonlinear fit model with least-squares regression without weighting was applied. Extra sum-of-squares F-tests were used to compare IC50 values (Figs. 1f and 3g and Extended Data Figs. 7e–g and 8f,g). For Extended Data Fig. 10i, statistics were derived from https://susztaklab.com/hk_genemap_kpmp/scRNA.
Comparisons were considered to be significant when P ≤ 0.05. If no significant difference between groups was detected, we did not indicate this specifically. Data were plotted as the mean ± s.d. if not specified otherwise.
All concrete P values are provided as Source data.
Reproducibility
All mice were strictly matched for weight, age and genetic background. Mice were selected into groups according to genotype and sex. Otherwise, they were selected randomly. All mouse experiments were conducted in a strictly double-blinded manner during data collection and analysis. Group sizes were planned ahead of experiments as required by German animal welfare regulations under strict application of 3R initiatives. During all of the experiments involving mammal biomaterials (mice, pigs, humans), investigators were blinded to experimental groups during data acquisition and analysis. No blinding was performed for cell culture assays.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.