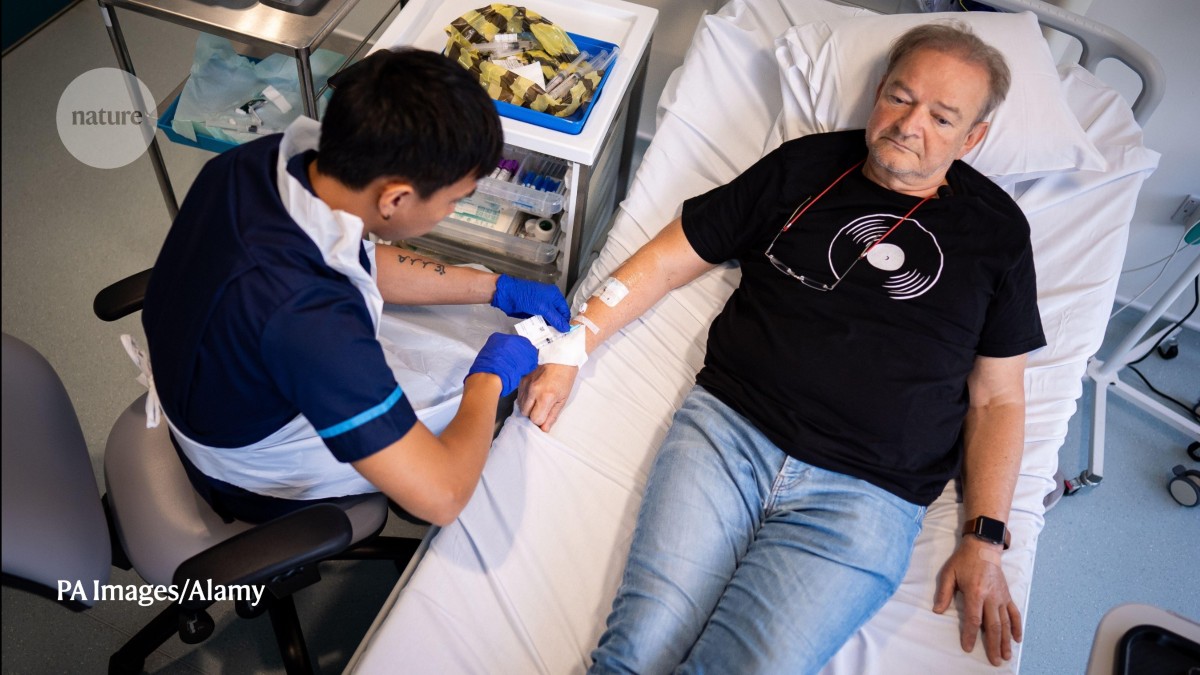
A patient at London’s UCL Hospital receives BioNTech’s mRNA immunotherapy for non-small-cell lung cancer, in the first UK clinical trial.Credit: PA Images/Alamy
The path to therapeutic cancer vaccines is strewn with disappointment. In the 20-year history of clinical trials for vaccines that are designed to treat existing cancer by stimulating the immune system to attack it, “all of them pretty much failed”, says Kavitha Yaddanapudi, an immunologist at the University of Louisville in Kentucky. Although two preventative cancer vaccines have been highly successful in targeting the viruses that cause liver and cervical cancer, stopping the spread of cancer that has already taken hold remains a significant challenge.
The theory behind therapeutic cancer vaccines is simple: research has shown that the immune system responds to cancer, which justified the idea of vaccines that can stall or halt the spread of the disease. “We are pretty sure that even at the very earliest initiation of cancer, the immune system gets involved,” says Olivera Finn, an immunologist at the University of Pittsburgh in Pennsylvania. But despite seeing signs of a positive immunological response to a cancer vaccine — such as increased levels of cancer-targeting immune T cells — in trial after trial, this rarely translated to any significant impact on tumours and patient outcomes.
Nature Index 2025 Cancer
A rare success story is the prostate cancer vaccine Provenge, which works by harvesting the patient’s own immune cells, exposing them to a prostate cancer-specific protein, then reinjecting them back into the patient to boost their immune response to the cancer. The treatment went to market in the United States in 2010 and has since been made available in several countries. It has been shown to extend the lives of people with advanced disease by around four months, but it does not slow disease progression.
As interest in therapeutic cancer vaccines waned, another treatment — an immunotherapy technique known as checkpoint blockade yielded far more promising results. This approach involves blocking proteins called immune checkpoints, which cancer cells use to switch off the body’s immune response. Checkpoint inhibitor medications have transformed the treatment and prognosis of cancers such as lung and melanoma, and shed light on why so many cancer vaccines have failed. “Cancer immunotherapy and cancer vaccines, they overlap a lot with the same end goal of activating your immune system to fight harder and smarter against the cancer,” Yaddanapudi says.
Now informed by insights from checkpoint inhibitors, the search for cancer vaccines has resumed with a spring in its step, and is targeting some of the deadliest cancers. But there’s a long road ahead, as early results deliver more questions than answers.
Timing vaccination
One approach that is showing great potential is personalized cancer vaccines. Vinod Balachandran, a surgical oncologist at the Memorial Sloan Kettering Cancer Center in New York, is working on a therapeutic vaccine to treat pancreatic cancer — an aggressive disease with a roughly 10% five-year survival rate.
Previous research by Balachandran and his colleagues1 found that patients who had survived for longer than five years showed a unique immunological imprint of the disease in their T cells. This prompted the question of whether immune recognition could be induced by a vaccine based on a patient’s own tumour cells. “Our finding had suggested that spontaneous immune recognition of a mutation can occur,” Balachandran says. “When such immune recognition occurs, it appears to impact the clinical outcome.”
A phase I trial was launched in 2019 for a therapeutic mRNA vaccine, which uses messenger RNA to instruct cells to produce tumour-specific proteins that trigger an immune response. The vaccines were ‘made to order’ for a small cohort of patients, based on tissue samples taken from their tumours. The tumours were removed in a New York hospital and shipped to a clinic in Germany within 72 hours, says Balachandran. “Within eight weeks of surgery, we received the patient’s individualized RNA vaccines, and then we administer it to the patient.”
The results2 have been mixed, but encouraging. Eight of 16 patients showed a strong immunological response to the vaccine and six of those had no recurrence of their cancer after three years. Six of the eight patients who did not show an immunological response experienced tumour recurrence.
One complication of the study is that the majority of those who didn’t show an immunological response had previously had surgery to remove their spleen — a routine, standard-of-care option in pancreatic surgery. The spleen was one of the targets for the vaccine delivery system, so one theory is that its removal might have affected the vaccine’s efficacy, says Balachandran. A phase II trial is under way in patients who have intact spleens.
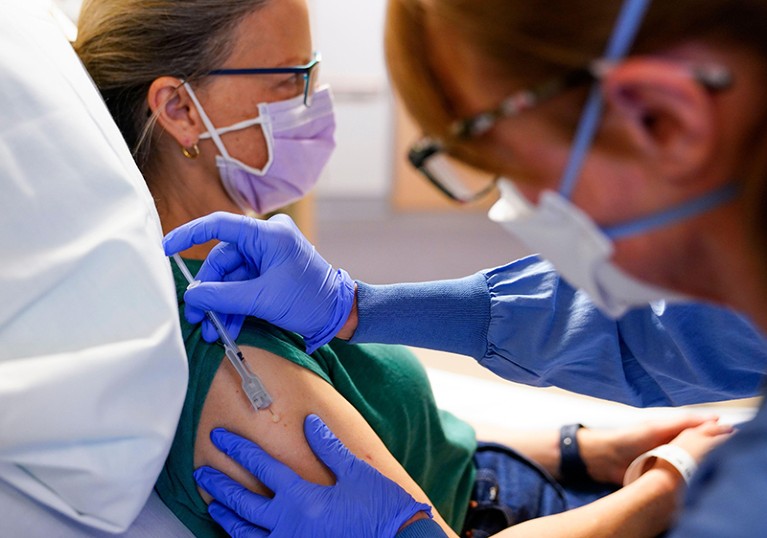
An experimental breast cancer vaccine at University of Washington Medical Center.Credit: Lindsey Wasson/AP/Alamy
This work highlights a key challenge in developing therapeutic vaccines: people receiving them already have cancer and are likely to have undergone a variety of treatments for it. However early the disease is found, “the immune system is affected because it has been dealing with this cancer and the cancer has put an imprint on it”, Finn says. This could be why so many therapeutic cancer vaccines have shown promise in animal models but then failed to show an effect in humans. “The animal models don’t have this long interplay between cancer and the immune system,” says Finn, whereas by the time a tumour is diagnosed in a human, the immune system might already be compromised too much for a vaccine to have any impact.
Finn is interested in what she calls ‘cancer interception’, which sits between pure therapy and pure prevention. Cancer interception might apply to individuals who are known to be at high risk of cancer, such as heavy smokers or those with family history or genetic predisposition to cancer. “With the new imaging technologies, we can really discover all sorts of premalignant lesions” before they turn into cancer, she says. “That’s the window of opportunity.”
Finding patients in this premalignant state isn’t easy. Finn and her colleagues have been investigating the use of a cancer vaccine that targets a protein called MUC1 — which is altered in many cancers — in people who have colorectal polyps that often progress to colorectal cancer. These are patients whose polyps will be removed, and then it’s a case of “watch and wait”, Finn says. But it offers a narrow window of opportunity to vaccinate and potentially prevent the emergence of more polyps or tumours.
A study3 by Finn and colleagues of this intervention in around 100 patients with a recent history of colorectal adenomas found a significant reduction in recurrence, but only among the 25% of vaccinated patients who showed an immunological response to the vaccination. The team is also conducting trials of the vaccine in smokers with hopes of reducing the risk of lung cancer, and in women who have been diagnosed with ductal carcinoma in situ, a very early and non-invasive form of breast cancer that can turn invasive if left untreated.
Choosing targets
Another key challenge for vaccines is selecting the right target. Tumour-associated antigens — proteins or molecules on the surface of cancer cells that trigger an immune response — are very difficult to pinpoint, for example. Not all cancers display a lot of them, for one thing, and they can also be found on healthy cells. Some are not detectable by the immune system at all. It’s also difficult to predict how or whether an antigen will provoke an immune response, says Siow Ming Lee, a medical oncologist at University College London, who is leading a clinical trial of an mRNA vaccine for lung cancer patients.