You have full access to this article via your institution.

Biochemists Georges Köhler (left) and César Milstein shared the 1984 Nobel Prize in Physiology or Medicine with Niels Jerne for their work on monoclonal antibodies.Credit: Bonn-Sequenz/ullstein bild/Getty, Bettmann/Getty
On 7 August 1975, Nature published a paper that has had some of the greatest impacts on science and health care in modern times. The study, by biochemists Georges Köhler and César Milstein, described a method for creating lab-made copies of antibodies — called monoclonal antibodies1. Antibodies produced by the immune system are specific to single, unique antigens — these are the molecules generated by viruses, bacteria and other foreign agents that invade the body and try to overpower its defences. Monoclonal antibodies can be produced in huge quantities and can be designed to recognize any target. They have become the workhorse for research laboratories. In the clinic, they are used to diagnose and treat a variety of conditions, including autoimmune diseases2, allergy3 and cancer4.
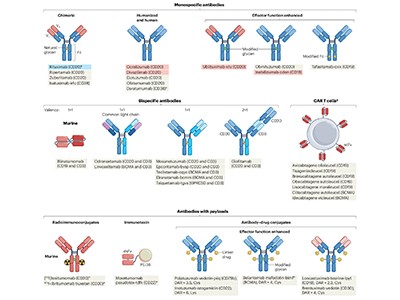
At least 212 antibody drugs have benefited tens of millions of people so far, write Andrew Chan, Greg Martyn and Paul Carter, researchers at Genentech in San Francisco, California, in an article in Nature Reviews Immunology on the past, present and future of antibody treatments5. The global market for monoclonal antibodies, worth around US$250 billion in 2024, is projected to double in value within five years (see go.nature.com/4muxakf).
Fifty years of monoclonals: the past, present and future of antibody therapeutics
Köhler and Milstein’s paper was the culmination of a worldwide effort to understand antibodies: their origin, structure, mechanism and role in the body’s immune system. This all happened without the complex organization and funding of today’s scientific consortia: at the time, large funders, notably the UK Medical Research Council (MRC) and the US National Cancer Institute, had a more hands-off role than they do now. This story shows that it is possible to establish a multibillion-dollar industry purely by letting scientists communicate their research, exchange information and share materials.
By the time Milstein arrived in the early 1960s at the MRC’s Laboratory of Molecular Biology (LMB) — then the world’s leading centre for studies of DNA structures — in Cambridge, UK, scientists had made progress in understanding antibodies’ basic structure. But they didn’t know how this class of proteins could target specific antigens.
Following up on biologist Joshua Lederberg’s theory that somatic mutations — changes in DNA not inherited from parents — underlie antibody diversity6, Milstein and fellow biochemist Sydney Brenner proposed a mechanism for restricting these mutations to specific gene regions7. As interest in this idea grew, so did the need to find ways to grow antibody-producing cells in the lab. Several groups succeeded in this step, which in turn allowed Milstein and his collaborators to investigate their theory experimentally in myeloma, a cancer of the white blood cells that are crucial to the immune response. It was hard work, not least because somatic mutations occur rarely. “The difficulty was that no one knew which specific antigens the myeloma cells bound to,” explains medical historian Lara Marks, who curated an exhibition on monoclonal antibodies for the MRC on the invention’s 40th anniversary.
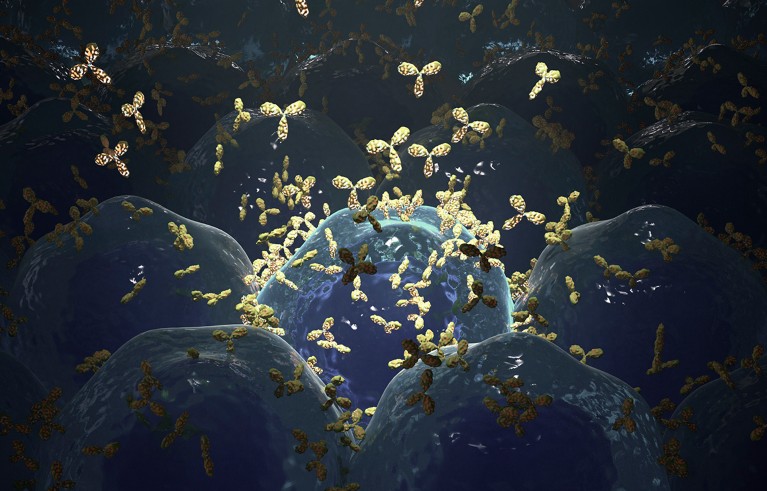
An illustration of the generation of monoclonal antibodies.Credit: NANOCLUSTERING/SPL
Milstein and Köhler solved this problem by immunizing mice with a particular antigen and taking healthy B cells from the animals’ spleens. This, they predicted, would trigger the production of an antibody specific to that antigen. The antibody-producing B cell was then fused with a mouse myeloma cell, creating a hybrid called a hybridoma. Using this technique, they were able to produce essentially limitless quantities of antibodies with known specificity.
PastCast: Monoclonal antibodies, from basic science to blockbuster drugs
Milstein’s lab received many requests for cell lines, which were dispatched to researchers all around the world, at little or no cost. This enabled other groups to replicate and build on the invention, creating the current industry. Some policymakers were concerned that the LMB and MRC were too slow to think about intellectual property. In retrospect, it’s an unfair criticism. The modern biotech industry was in its nascent phase and it would be several years before the United States (and United Kingdom) would allow universities to patent scientific knowledge from government-funded research. The many groups involved in the breakthrough were mainly looking to answer a fundamental scientific question and did so according to the research community’s practices at the time.
The colossal industry that has grown around monoclonal antibodies continues to expand, and scientists are now looking to answer new questions, such as how to deploy artificial-intelligence technologies to design antibodies with drug-like properties — a task that is more complicated than using such tools to predict protein structures.
The story of monoclonal antibodies illustrates how an industry that changed health care was born through a scientific ‘relay race’. Fifty years later, the scientific landscape has transformed, in many ways for the better. Funders and policymakers, however, should consider whether breakthrough discoveries are aided by time-limited funding and complicated management structures. But stakeholders should not lose sight of perhaps the most important aspect of the work: free exchange of knowledge and materials pays dividends.