Animals
Cpeb4 KO mice45 and conditional transgenic mice overexpressing the human CPEB4 isoform that lacks exonâ4 (TgCPEB4Î4)2 both in a C57BL/6J background were used. All mice were bred and housed in the CBMSO animal facility. Mice were grouped four per cage with food and water available ad libitum and maintained in a temperature-controlled environment on a 12â12âh lightâdark cycle with light onset at 8:00 and a relative humidity of 55â±â10%. Animal housing and maintenance protocols followed local authority guidelines. Animal experiments were performed under protocols approved by the CBMSO Animal Care and Utilization Committee (Comité de Ãtica de Experimentación Animal del CBMSO, CEEA-CBMSO) and Comunidad de Madrid (PROEX 247.1/20).
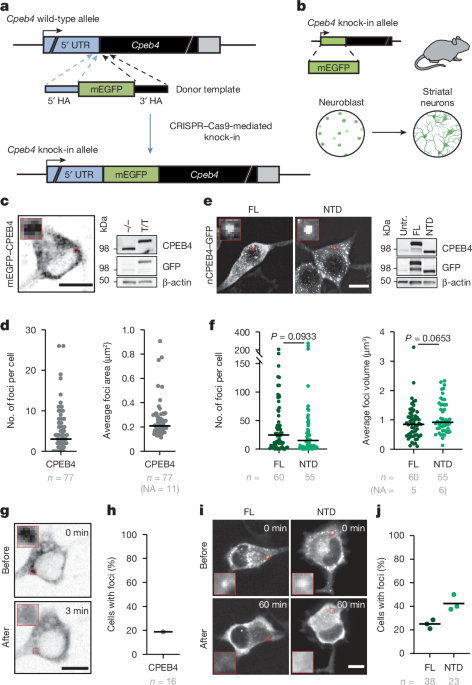
Generation of mEGFPâCPEB4 mice
A synthetic sequence consisting of the mEGFP linker sequence flanked by short regions of 5â² homologous (238âbp) and 3â² homologous (99âbp) DNA was obtained (Twist Bioscience). PCR was carried out on the synthetic sequence using the following primers: Fw ssDNA-mGFPâCPEB4 (phosphorylated) tacttcaagcaaacatatttgagatacagggga; Rv ssDNA-mGFPâCPEB4 (thiol-protected) GGTGATGGTGTGGAGGCTGC. Single-stranded DNA (ssDNA) was generated from double-stranded DNA by lambda exonuclease digestion of the phosphorylated strand, followed by gel purification and column extraction. Animals were generated by electroporation of isolated mouse zygotes with ssDNA combined with Cas9 protein and guide/tracr RNA ribonuclear protein complexes (guide; C45gRNA ATCCTAAAAATAATAAATGG). The correct integration of the knock-in cassette was confirmed by PCR and sequencing of the region. The resulting positive mice were crossed with C57BL6/J mice to confirm germline transmission. The offspring were maintained in a C57BL/6J background, and routine genotyping was performed by PCR using the following genotyping primers: 5â²-ACGTAGGGTGATAAGCTGTGAT–3â² (Fw) and 5â²-AGGGTCTTGTTGTTCTTGCTGT-3â² (Rv). Mice were maintained in a specific pathogen-free facility with a 12â12âh lightâdark cycle at 21â±â1â°C at a relative humidity of 55â±â10% and given ad libitum access to standard diet and water. Animal handling and all experimental protocols were approved by the Animal Ethics Committee at the Barcelona Science Park and by the Government of Catalonia.
Mouse mEGFPâCPEB4 striatal neuron extraction and culture
mEGFPâCPEB4 mice over 6âweeks of age were crossed in timed matings. Females were weighed weekly to monitor gestation progression. Females with an increment over 3âg up to 18âdays after a positive plug were euthanized and embryos were collected at embryonic dayâ18.5 in cold buffer containing 1à HBSS, 10âmM glucose and 10âmM HEPES. A tail sample was also collected for embryo genotyping. Brains were dissected in the aforementioned buffer on an ice-cold plate, and the striatum was extracted and chopped. Samples were centrifuged and digested with a previously heated solution containing 1à HBSS, 10âmM glucose, 10âmM HEPES, 12âUâmlâ1 papain (Worthington LS003180) and 5âmM l-cysteine for 15âmin at 37â°C. Samples were then disaggregated in a buffer containing 1à DMEM/F-12, 2âmM glutamine, 1âmM sodium pyruvate, 20âmM glucose and 10% inactivated horse serum. Cells were seeded at a confluence of 25,000 cells per well in µ-Slide 8-well ibiTreat imaging plates (Ibidi, 80826) previously coated with poly-d-lysine. Cells were then incubated at 37â°C for 1âh. After this time, medium was exchanged with previously tempered medium containing 1à Neurobasal (Gibco, 21103049), 1à B27 with vitaminâA (Gibco, 17504044), 2âmM glutamine and 0.5% penicillinâstreptomycin (PS). Medium was refreshed every 2â3âdays. Neurons were considered differentiated after 7âdays of culture. After genotyping, homozygous mEGFPâCPEB4 mice and wild-type littermates were selected for imaging. When specified, cell depolarization was induced by the addition of 50âmM KCl with 1:3 medium dilution. Neurons were maintained in culture for up to 14âdays.
mEGFPâCPEB4 distribution in neurons
Primary striatal neurons from mEGFPâCPEB4 mice were imaged at 7âdays of differentiation using a LIPSI spinning disk microscope (Nikon). Image acquisition was performed using a fully incubated, high-content, high-speed screening LIPSI platform (Nikon) equipped with an Eclipse Ti2 inverted microscope and a Yokogawa W1 confocal spinning disk unit. The spinning disk unit with an Apo LWD Ã40 water lens of 1.15 numerical aperture, and a 488ânm (20%) laser was used for acquisition on a Prime BSI Photometrics sCMOS camera. NIS Elements AR (v.5.30.05) software was used for acquisition, and Fiji/ImageJ software was used to adjust images for visualization.
mEGFPâCPEB4 neuronal stimulation with NMDA
Primary striatal neurons from mEGFPâCPEB4 mice were imaged at 14â21âdays of differentiation, and where specified, neuron stimulation was induced by the addition of 20âµM NMDA (Tocris, 0114), a selective NMDA receptor agonist. Stimulated neurons were imaged using a fully incubated Zeiss Elyra PS1 LSM 880 confocal microscope with a Plan ApoChromat Ã63/1.2 Imm corr oil objective. A 488ânm (50%) laser was used for acquisition on a Prime BSI Photometrics sCMOS camera. Images were captured every 15âmin over the recording period. Zen Elements AR (v.5.30.05) software was used for acquisition, and Fiji/ImageJ software was used for image quantification and to adjust images for visualization. A tailor-made macro applying an intensity threshold was used to accurately segment cytoplasmic condensates and the whole cell for each time frame. The condensed fraction per frame was obtained as the sum of areas of condensates divided by the cell area. For representation, the values were normalized to that measured at the end of the stimulation period.
nCPEB4 extraction from mouse brains
To extract nCPEB4 from the brains of 6-month-old control mice, Cpeb4 KO mice and TgCPEB4Î4 mice, around 500âmg of tissue was first collected and snap-frozen in liquid nitrogen. Each sample was homogenized in 5âml lysis buffer (50âmM Tris, pHâ7.7, 5% glycerol, 0.1% Triton X-100, 1% NP-40, 50âmM NaCl, 50âmM imidazole and Pierce protease inhibitor, EDTA-free) using a Polytron homogenizer and rotated for 30âmin at 4â°C. The homogenate was moved to high-speed PPCO centrifuge tubes and centrifuged at 48,000g at 4â°C for 20âmin. After this, the supernatant was retained while the resultant pellet was dissolved in 4âml lysis buffer and homogenized further using the Polytron homogenizer with the same protocol. This process was repeated 3 times (with 1âml reduction of lysis buffer after each round of homogenization) to maximize the extraction of nCPEB4. To further clarify the combined supernatants, they were filtered through a Miracloth membrane (Millipore) to remove lipids and then passed through a 0.45âμm filter. Exploiting the histidine-rich regions present in the sequence of nCPEB4 (23RFHPHLQPPHHHQN36 and 229LSQHHPHHPHFQHHHSQHQQ248), a 2-elution step Ni2+-affinity chromatography was carried out using a Histrap HP 5âml (GE Healthcare) on a FPLC apparatus (ÃKTA Pure, GE Healthcare). The combined supernatants were injected into a column pre-equilibrated with binding buffer consisting of 50âmM Tris, pHâ7.7, 50âmM NaCl and 50âmM imidazole. The bound fraction was initially washed in a high-salt buffer consisting of 50âmM Tris, pHâ7.7, 1âM NaCl and 50âmM imidazole. This step is crucial as it removes a high-molecular-weight nonspecific binder that was detectable by western blotting (WB), even in the Cpeb4 KO mice. The removal of this nonspecific binder is important to ensure the integrity of subsequent analyses. Once the nonspecific binder was completely removed, nCPEB4 was eluted using a second buffer containing 50âmM Tris, pHâ7.7, 50âmM NaCl and 500âmM imidazole. The non-bound, washed and eluted fractions were then analysed by WB using Bis-Tris 4â12% gradient gels. At this point, fractions containing nCPEB4, verified by WB in SDSâPAGE, were used for proteinaseâK digestion and SDS-resistance analysis using 1.5% SDDâAGE as previously described46. Here, the non-boiling proteins were transferred to a nitrocellulose membrane by capillary methods and probed with an anti-CPEB4 antibody. Samples identified as CPEB4 monomers and aggregates were selected for subsequent seeded aggregation assay. Additionally, to quantify the amount of CPEB4 aggregates, the same samples were injected into a Superdex 200 Increase 10/300 GL (GE Healthcare) column pre-equilibrated with 1à PBS, pHâ7.5. The eluted fractions from the gel filtration chromatography were combined every 4 fractions (2âml) and concentrated into 100âµl using a Pierce concentrator, PES, 30âK MWCO, 0.5âml, to be ultimately analysed using 1.5% SDDâAGE. For all the blots described here, including SDSâPAGE, immunodot blots and SDDâAGE, a 1:2,000 dilution of polyclonal rabbit anti-CPEB4 antibody (Abcam, ab224162) and a 1:5,000 dilution of HRP-linked anti-rabbit IgG antibody (Cell Signaling Technology, CST-7074S) were used.
ProteinaseâK digestion
About 400âng of total protein, estimated from BCA assays, containing endogenous nCPEB4 extracted from the brains of 6-month-old control mice, Cpeb4 KO mice and TgCPEB4Î4 mice were digested with 0.1, 0.5, 1, 5 and 10âng of proteinaseâK for 2âmin at 37â°C. ProteinaseâK activity was stopped by heating the samples to 75â°C. Next, 2âμl of the enzyme-treated reaction mixture was manually applied to a nitrocellulose membrane. The membrane was blocked in 5% milk in TBS-T buffer and probed with anti-CPEB4 antibody (Abcam, ab224162).
Seeded aggregation assay
A concentration of 1% w/w of the seed, 40âng of total protein containing nCPEB4 aggregates extracted from TgCPEB4Î4 mouse brains, was incubated with the substrate, which consisted of 4âμg total protein containing soluble nCPEB4 extracted from wild-type (WT) mouse brains. Unless concentrated 100-fold, the seed used was not detectable by WB. The seeding reaction was carried out at 4â°C for 24âh during the time course experiment in 50âmM Tris, pHâ7.7, and 50âmM NaCl. The seeded reactions, which were non-boiled, were analysed using 1.5% SDDâAGE as previously described46. The proteins were transferred to a nitrocellulose membrane by capillary methods and probed with anti-CPEB4 antibody (Abcam, ab224162) to follow the aggregation of WT nCPEB4 in a time-dependent manner.
Proteostat staining and CPEB4 immunofluorescence
Six-week-old TgCPEB4Î4 mice (nâ=â4) and control littermates (nâ=â3) were anaesthetized by an intraperitoneal injection of pentobarbital and then transcardially perfused with PBS. Brains were immediately removed and each hemisphere placed in 4% paraformaldehyde overnight at 4â°C, followed by 3 PBS washes (10âmin each) and then immersed in 30% sucrose in PBS for 72âh at 4â°C and them included in optimum cutting temperature compound (Tissue-Tek, Sakura Finetek Europe, 4583) and immediately frozen. Samples were stored at â80â°C until use.
Brain hemispheres were cut sagittally at 30âµm on a cryostat (Thermo Scientific), and sections were stored (free floating) in glycol-containing buffer (30% glycerol and 30% ethylene glycol in 0.02âM PB) at â20â°C.
For staining, sections (2 per mouse) were washed in PBS to eliminate the cryoprotective buffer and permeabilized in 0.2% Triton X-100 for 30âmin at room temperature, and then stained with the dye Proteostat (Enzo51035-K100; 1:2,000) for 15âmin at room temperature followed by 2 PBS washes (10âmin each) and then 1% acetic acid for 30âmin at room temperature, followed by 3 PBS washes (10âmin each). For CPEB4 immunofluorescence, sections were immersed in blocking solution (2% NGS, 1% BSA and 0.2% Triton X-100 in PBS) for 1âh at room temperature and then incubated overnight at 4â°C with anti-CPEB4 primary monoclonal antibody (1:1,000, mouse monoclonal, homemade, ERE149C) in blocking solution. After 3 PBS washes (10âmin each), sections were incubated with Alexa 488 donkey anti-mouse secondary antibody (1:500, Thermo Fisher, A-21202) for 1âh followed by 3 PBS washes (10âmin each) and, finally, nuclei were stained by incubating with DAPI (1:10,000 in PBS, Merck) followed by 3 PBS washes (10âmin each) and mounted with Prolong medium (Life Technologies).
Images of the striatum were obtained with a vertical Axio Observer.Z1/7 laser scanning microscope (LSM 800, Carl Zeiss) at Ã63 magnification with Ã2 optical zoom and analysed by performing zâstacks (11 optical sections with a thickness of 1âμm, spanning 6.6âμm on the zâaxis). Sequential scanning mode was used to avoid crosstalk.
Medium-sized spiny neurons were distinguished by the morphology and size of the nucleus, and fields were selected to typically include 4â8 medium-sized spiny neurons. The number of Protesotat and CPEB4 double-positive foci was manually counted per cell fully included within the zâstack. Typically, between 16 and 20 cells were analysed per mouse from a total of 50 control and 73 TgCPEB4Î4 neurons.
Significance for differences in the number of positive foci between control mice and TgCPEB4Î4 mice was assessed using a generalized linear mixed model (familyâ=âPoisson(linkâ=ââidentityâ)) with mouse as the random effect (Supplementary Methods).
Plasmids for expression in N2a cells
Human nCPEB4 (UniProt identifier Q17RY0-1) FL open reading frame (ORF) was cloned into a pBSK vector. The me4 sequence (nucleotides 1258â1281) was deleted by PCR on a pBSK-nCPEB4 plasmid using Gibson assembly master mix (New England Biolabs, E2611S) following the manufacturerâs instructions. Mutagenesis of nCPEB4 phosphorylation sites was performed using a QuikChange Lightning Multi Site-Directed Mutagenesis kit (Agilent Technologies, 210513), with oligonucleotides purchased from Sigma-Aldrich, following the manufacturerâs instructions. ÎHC mutants were generated by PCR mutagenesis on a pBSK-nCPEB4 plasmid, with oligonucleotides purchased from Sigma-Aldrich. For cell transfection, nCPEB4 ORF, FL, NTD and mutants were cloned into pPEU4 and pPEU5 vectors, which contain a C-terminal eGFP or mCherry tag, respectively, by In-Fusion (BD Clontech) cloning reaction47. For BioID, xCPEB4 or BirA ORF was cloned into a pBSK vector. me4 was added to the xCPEB4 sequence by PCR on a pBSK-xCPEB4 plasmid using Gibson assembly master mix (New England Biolabs, E2611S) and following the manufacturerâs instructions. A MYCâtag and BirA ORF were added at the Nâterminus of pBSK-xCPEB4 plasmid. For competition experiments, xCPEB1 RRMZZ and xCPEB4 RRM domains were cloned in pBSK, and a HAâtag was added at the Nâterminus. pBSK-Emi2 3â²âUTR was obtained from a previous study48.
N2a cell culture, differentiation and DNA transient transfection
N2a cells were grown in DMEM with 10% FBS, 1% PS and 2âmM l-glutamine for maintenance. For fixed-cell imaging, cells were seeded on 6-well plates with 12-mm-diameter poly-lysine-coated glass coverslips (Marienfeld Superior). For live-cell imaging, cells were seeded on µ-Slide 8-well ibiTreat plates. For differentiation, medium was exchanged with DMEM with 0.5% FBS, 1% PS, 2âmM l-glutamine and 1âµM retinoic acid and cells were grown for 48âh. They were then transfected at 60% confluence with 1.25âµg DNA using Lipofectamine LTX and Plus reagent (Thermo Fisher, 15338100) following the manufacturerâs protocol. When specified, N2a depolarization was induced as described for striatal neurons, specified in the section âMouse mEGFPâCPEB4 striatal neuron extraction and cultureâ.
N2a cell line characterization
N2a cells, like neurons, express CPEB4 variants including and excluding me4 (nCPEB4 and nCPEB4Î4, respectively), independently of their differentiation status (Extended Data Fig. 1e). By contrast, cell lines of non-neural origin only express nCPEB4Î4. Inclusion of me4 in N2a cells correlates with the expression of the splicing factor SRRM4 but not with that of RBFOX1 (Extended Data Fig. 1f). Depletion of SRRM4 in N2a cells decreases the inclusion of me4, whereas overexpression of SRRM4 in the non-neuronal cell line 293T forces its inclusion5,49 (Extended Data Fig. 1g). N2a cells, therefore, recapitulate the neuron-specific regulation of CPEB4 alternative splicing.
nCPEB4âGFP distribution in N2a cells
Twenty-four hours after transfection, N2a cells were fixed with 4% paraformaldehyde (Aname, 15710) in PBS for 10âmin at room temperature. They were then washed with PBS and incubated with 0.5âµgâµlâ1 DAPI (Sigma) for 15âmin. Coverslips were rinsed with PBS and mounted on a glass slide with Prolong Gold Antifade mountant (P36934, Invitrogen). Image acquisition was performed with a Leica SP5 confocal microscope (Leica Microsystems), and zâseries stacks were acquired at 1,024âÃâ1,024 pixels using a Ã63/1.4 numerical aperture oil immersion objective with a zoom factor of 2. Argon 488ânm (20%) and diode 405ânm (10%) lasers were used. Hybrid detectors for GFP (500â550ânm with 33% gain) and DAPI (415â480ânm, 33% gain) were used for acquisition. LAS AF Leica software was used to acquire 10â20 zâstack slices per cell with a zâstep size of 0.5âµm. Fiji/ImageJ software was used to perform the image analysis. A tailor-made macro using BioVoxxel Toolbox and 3D object counter plug-ins was used to accurately segment and obtain the number and volume of foci per cell.
Live-cell imaging of GFP-tagged CPEB4 variants in N2a cells
Live imaging of overexpressed nCPEB4âGFP variants in N2a cells was performed 20âh after transfection, whereas primary striatal neurons from mEGFPâCPEB4 mice were imaged at 7âdays of differentiation. For both types of cells, image acquisition was performed using a spinning disk microscope (Andor Revolution xD, Andor). A total of 24 images were taken per experiment (4 before the addition of the stimulus and 20 after), with 13 zâstacks at 512âÃâ512 pixels of format resolution. Images were acquired with a step size of 0.5âμm. For acquisition, the typical frame rate was adjusted to 5 images perâs at 50âms integration time of the EMCCD camera (Andor). An argon 488ânm laser (20%) was used for acquisition with a 1.4 numerical aperture/Ã60 oil immersion objective. Fiji/ImageJ software was used to obtain a zâprojection of the zâstacks and subsequent concatenation of images. The obtained time-lapse images were subsequently used for manual quantification of nCPEB4 dissolution events. Cells were manually classified into two categories depending on the existence of cytoplasmic foci at tâ=â60âmin: cells with remaining foci or cells without. For nCPEB4 and nCPEB4Î4 FL comparison, the percentage of cells with remaining cytoplasmic foci after the depolarizing stimuli (tâ=â60âmin) was calculated from a pool of 7 experiments. Unless specified, the percentage of cells with remaining cytoplasmic foci after the depolarizing stimuli (tâ=â60âmin) was calculated per each experiment. When specified, blind analysis and classification were performed independently by a group of four different people from a pool of experiments.
FRAP in N2a cells
A spinning disk microscope from Andor, equipped with a FRAPPA module, was used for FRAP experiments. A total of 350 images were taken per experiment (50 images before the bleaching and 300 after) at 512âÃâ512 pixels. The typical frame rate was set to the fastest (88âms) with an exposure time of 50âms on an EMCCD camera. An AOTF 488ânm laser (20%) was used for acquisition, and 50% laser intensity was set for bleaching in 2 repeats with a dwell time of 40âms. Fiji/ImageJ software was used for FRAP analysis. Three regions of interest (ROIs) were defined per video: background, cell and bleaching area. The mean fluorescence intensity was obtained for the 3 ROIs for all 350 frames, and the output was exported in tabular format. Outputs were then entered on the easyFRAP website50. Full-scale normalization was selected, âinitial values to discardâ was set to 20 and the curves obtained were fitted to a single exponential model. Fluorescence recovery curves, mobile fraction and half time of recovery were obtained for each experimental condition.
Mapping of nCPEB4 post-translational modified sites by mass spectrometry
Overexpressed nCPEB4âGFP and nCPEB4Î4âGFP were immunoprecipitated from basal (âstim) and stimulated (+stim) N2a differentiated cells. Cells were lysed in ice-cold RIPA buffer containing 50âmM Tris HCl pHâ8, 1% Nonidet P-40 (NP40), 0.1% SDS, 1âmM EDTA, 150âmM NaCl, 1âmM MgCl2, 1à EDTA-free complete protease inhibitor cocktail (Roche, 5056489001) and phosphatase inhibitor cocktails (Sigma, P5726 and P0044). Cells were subsequently sonicated for 5âmin at low intensity with a standard bioruptor diagenode. Following centrifugation (4â°C for 10âmin at maximum speed), supernatants were collected, precleared and immunoprecipitated overnight at 4â°C with 50âμl GFP-conjugated Dynabeads proteinâA (Invitrogen). Beads had previously been conjugated with 5âμl anti-GFP antibody (Invitrogen, A6455) diluted in 500âμl PBS 1à for 2âh at room temperature. After immunoprecipitation, beads were washed with cold RIPA buffer and eluted with Laemmli sample buffer. Eppendorf LoBind microcentrifuge tubes (Eppendorf, 30108116) were used for the entire protocol. The immunoprecipitated elutions were run on precast 4â20% gradient gels (Midi Criterion TGX, Bio-Rad) and stained with Coomassie blue for 1âh at room temperature. Bands at the expected nCPEB4âGFP molecular weight were cut, washed with 50âmM NH4HCO3 and acetonitrile, reduced with 10âmM DTT and alkylated with 50âmM IAA. Samples were digested with trypsin and digestion was stopped by the addition of 5% formic acid. Following evaporation, samples were reconstituted in 15âμl of 1% formic acid and 3% acetonitrile. Mass spectrometry analysis of nCPEB4 PTM sites was performed as previously described7 with some modifications. In brief, samples were loaded in a μ-precolumn at a flow rate of 250ânlâminâ1 using Dionex Ultimate 3000. Peptides were separated using a NanoEase MZ HSS T3 analytical column with a 60âmin run and eluted with a linear gradient from 3 to 35% bufferâB in 60âmin (bufferâA: 0.1% formic acid in H2O; bufferâB: 0.1% formic acid in acetonitrile). The column outlet was directly connected to an Advion TriVersa NanoMate (Advion) fitted on an Orbitrap Fusion Lumos Tribrid (Thermo Scientific). Spray voltage in the NanoMate source was set to 1.7âkV. The mass spectrometer was operated in a data-dependent acquisition mode. Survey mass spectrometry scans were acquired in the orbitrap with the resolution (defined at 200âm/z) set to 120,000. The top speed (most intense) ions per scan were fragmented in the HCD cell and detected in the orbitrap.
For peptide identification, searches were performed using MaxQuant (v.1.6.17.0) software and run against a target and decoy database to determine the false discovery rate. The database included proteins of interest sequences (nCPEB4âGFP and nCPEB4Î4âGFP) and contaminants. Search parameters included trypsin enzyme specificity, allowing for two missed cleavage sites, oxidation in methionine, phosphorylation in serine, threonine and tyrosine, methylation and demethylation in lysine and arginine, and acetylation in the protein Nâterminus as dynamic modifications, and carbamidomethyl in cysteine as a static modification. Peptides with a qâvalue lower than 0.1 and false discovery rateâ<â1% were considered as positive identifications with a high confidence level. Mass spectrometry spectra were searched against contaminants (released in 2017) and user proteins using Andromeda and MaxQuant (v.1.6.17.0) software. To accept a site as modified, PTM localization probability was set above 75%. For the differential expression analysis, a t-test on PTM site intensities from MaxQuant was applied for each site within nCPEB4 variants. For data visualization, two parameters were used for each PTM site, namely the sum of intensities of modified peptides that contain the specific PTM-site (Int_mod) and the PTM-to-base ratio, with the latter calculated as: Int_mod/Int_unmod, where Int_unmod is the sum of intensities of unmodified peptides that contain the site. For data visualization, the PTM-to-total ratio was calculated for each site as follows: PTM-to-totalâ=âInt_mod/(Int_modâ+â(Int_mod/PTM-to-base)).
Effect of phosphorylations on condensate dissolution
To strengthen the conclusion that phosphorylation of nCPEB4 does not promote condensate dissolution, we studied the behaviour in N2a cells of the condensates formed by phosphomimicking (S/T to D) and non-phosphorylatable (S/T to A) variants of nCPEB4(NTD), the phosphorylation status of which cannot be altered by depolarization. In agreement with our previous findings11, the former had a lower propensity to condense (Extended Data Fig. 2c) and, in agreement with our conclusion, both variants dissolved after depolarization (Extended Data Fig. 2d,e).
Intracellular pH tracking
Quantitative determination of intracellular pH (pHi) was performed using the cell-permeant ratiometric pH indicator SNARF-5F 5-(and-6)-carboxylic acid AM (Thermo Fisher) in live imaged N2a cells at 48âh of differentiation. In brief, for loading the pH indicator into cells, they were incubated with 10âµM SNARF-5F 5-(and-6)-carboxylic acid AM diluted in serum-free DMEM for 15âmin at 37â°C. Cells were then washed and imaged in serum-free DMEM. A Zeiss Elyra PS1 LSM 880 confocal microscope using a Plan ApoChromat Ã40/1.2 Imm corr DIC M27 water objective was used for acquisition at 2 emission wavelengths: â575ânm and 640ânm. Images were captured every 30âs over the recording period. pHi estimation was performed as described in previous publications51. In brief, in vivo pHi calibration was performed by fixing the pHi between 5.5 and 7.5 with a commercially available intracellular pH calibration buffer kit (Thermo Fisher). Valinomycin and nigericin were used to equilibrate the intracellular pH. The intensity of fluorescence emitted at the two wavelengths was used to calculate a ratio (RF640/F575) that is proportional to pHi. Fluorescence ratio values (RF640/F575) from cells with fixed pHi were used to obtain a calibration curve for each biological replicate. Experimental pHi estimation from the fluorescence ratio values was calculated using the following equation: pHiâ=â(RF640/F575â+âb)/m, where m is the slope from the calibration curve equation and b is the intercept.
RNA extraction and real-time quantitative RTâPCR
For N2a RNA extraction, cells were scraped into an ice-cold plate, collected and centrifuged at 500g for 5âmin at 4â°C. For mouse tissue RNA extraction, organs were ground with a liquid-nitrogen-cooled mortar to obtain tissue powder. Total RNA was extracted from both cells and tissue powder using TRIsure reagent (Bioline, Ecogen, BIO-38033) following the manufacturerâs protocol and using phenolâchloroform. The RNA concentration was determined using a Nanodrop spectrophotometer (Nanodrop Technologies). Next, 1âμg of total RNA was reverse transcribed using RevertAid reverse transcriptase (Themo Fisher, EP0442) following the manufacturerâs recommendations and using oligodT and random hexamers as primers. Quantitative real-time PCR (qPCR) was performed in triplicate in a QuantStudio 6flex (Thermo Fisher) using PowerUp SYBR green master mix (Thermo Fisher, A25778). All quantifications of mRNA levels were first normalized to an endogenous housekeeping control (Tbp), and then mRNA relative quantities to a reference sample (brain, N2a undifferentiated) were calculated using the 2âÎÎCt method. The following primers were used for qPCR: 5â²-TGATTCCATTAAAGGTCGTCTAAACT-3â² (Fw) and 5â²-GAAACAATGAAGACTGACCTCTCCTT-3â² (Rv) for Mm Cpeb4 isoform containing exonsâ3 and 4; 5â²-TGATTCCATTAAAGCAAGGACTTATG-3â² (Fw) and 5â²-GCTGTGATCTCATCTTCATCAATATC-3â² (Rv) for Mm Cpeb4 isoform lacking exonâ3; 5â²-TGATTCCATTAAAGGTCGTCTAAACT-3â² (Fw) and 5â²-GGAAACAATGAAGACTGACCATTAAT-3â² (Rv) for Mm Cpeb4 isoform lacking exonâ4; 5â²-ATTCCATTAAAGGTCAGTCTTCATTG-3â² (Fw) and 5â²-GCTGTGATCTCATCTTCATCAATATC-3â² (Rv) for Mm Cpeb4 isoform lacking exonsâ3 and 4; 5â²-GGAAAGGGACCTTCAAAGCAGT-3â² (Fw) and 5â² CTCTGTCCTTGGCATCGGCT-3â² (Rv) for Mm Srrm4; and 5â²-ACTTCTATGCAGGCACGGTG-3â² (Fw) and 5â²-AGCCAGGCATTGCAGAAGTAT-3â² (Rv) for Mm Rbfox1. Mm JunB and cFos primers were obtained from a previous study5.
Real-time semi-quantitative RTâPCR
For CPEB4 splicing isoform amplification, specific primers were used in CPEB4 exonâ2 (Fw primer) and exonâ5 (Rv primer) as previously described2. PCR products conforming to the 4 isoforms of CPEB4 were resolved on a 2% agarose/GelRed gel run at 130âV for 2âh.
WB analysis
Cells were lysed in ice-cold buffer containing 1% NP40, 150âmM NaCl, 50âmM Tris HCl (pHâ7.5), 2âmM EDTA, 2âmM EGTA, 20âmM sodium fluoride, 2âmM PMSF, 2âmM sodium orthovanadate, 1âmM DTT and 1à EDTA-free complete protease inhibitor cocktail. Lysed samples were then sonicated at medium intensity for 5âmin with a standard bioruptor diagenode, and total protein content was quantified using a DC Protein assay (Bio-Rad, 5000113). Next, 15â30âμg of total protein lysate was resolved on SDSâPAGE gels and transferred to a nitrocellulose membrane (Cytiva, 10600001). After 1âh of blocking at room temperature in 5% non-fat milk, membranes were incubated overnight at 4â°C with primary antibodies and subsequently with secondary antibodies for 2âh at room temperature. Specific proteins were labelled using the following primary antibodies: CPEB4 (1:100, homemade, mouse monoclonal, ERE149C); GFP (1:2,000, Invitrogen, rabbit polyclonal, A6455); MYC (1:1,000, Abcam, goat polyclonal, ab9132); and β-actin (1:10,000, Abcam, mouse monoclonal, ab20272) or streptavidin (1:5,000, Thermo Fisher, S911). The following secondary antibodies were used: goat anti-mouse (1:300, Thermo Fisher, 31430); goat anti-rabbit (1:300, Thermo Fisher, G-21234); and donkey anti-goat (1:300, Abcam, ab6885). Membranes were then incubated for 3âmin with Amersham ECL TM WB detection reagents (Sigma, GERPN2106) or for 5âmin with Clarity Western ECL substrate (Bio-Rad, 1705061).
nCPEB4 and nCPEB4Î4 co-localization experiments in N2a cells
mCherry red signals were acquired with a DPSS 561 excitation laser (9%) and a HyD2 detector set to 570â650ânm with a gain of 33%. DAPI and GFP signals were acquired using the settings specified in the section ânCPEB4âGFP distribution in N2a cellsâ. For measuring the extent of co-localization between the two channels, the ImageJ JaCoP plug-in was used to obtain Pearsonâs correlation coefficients and Manderâs overlap coefficients per cell.
Immunohistochemistry
Mouse embryos at embryonic dayâ13.5 were fixed in 10% neutral-buffered formalin solution and embedded in paraffin. Rabbit polyclonal primary antibody anti-CPEB4 (Abcam, ab83009) was used at 1:1,000 dilution. Embryo sections were counterstained with haematoxylin.
X.
laevis oocyte preparation
Stage VI oocytes were obtained from full-grown X.âlaevis females as previously described52. In brief, ovaries were treated with collagenase (2âmgâmlâ1; StemCell Technologies) and incubated in modified bath saline 1Ã medium with 0.7âmM CaCl2. Animal handling and all experimental protocols were approved by the Animal Ethics Committee at the Barcelona Science Park and by the Government of Catalonia.
X.
laevis BioID
BioID was performed as previously described7. In brief, 150 stage VI X.âlaevis oocytes were microinjected with 50.6ânl of 50ângâμlâ1 in vitro transcribed and polyadenylated RNAs corresponding to MYC-BirA-xCPEB4 variants. Oocytes were then incubated in 20âμM biotin (Merck) at 18â°C for 40âh. Oocytes were lysed in cold lysis buffer and centrifuged twice at 16,000g at 4â°C for 15âmin. Cold BioID lysis buffer was added to cleared extract, and the resulting mixture was subjected to clearing with PD MiniTrap G-25 columns (GE Healthcare). Next, 1.6% Triton X-100 and 0.04% SDS were added, and extracts were incubated with MyOne Dynabeads Streptavidin C1 (Invitrogen). The beads were then washed with a subsequent sequence of wash buffers. The beads were resuspended in 3âM urea, 50âmM NH4HCO3, pHâ8.0, and 5âmM DTT for 1âh at room temperature with orbital shaking and subsequently incubated in 10âmM iodoacetamide for 30âmin at room temperature, and then DTT was added. Proteins were on-bead digested with trypsin (Promega) at 37â°C for 16âh with orbital shaking. Digestion was stopped by the addition of 1% formic acid. Mass spectrometry analysis of biotinylated proteins in xCPEB4 and xCPEB4â+âEx4 was carried out at the Mass Spectrometry Facility at IRB Barcelona as previously described7. In brief, samples were analysed using an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Scientific). The MS/MS spectra obtained were searched against the UniProt (Xenopodinae, release 2017_02) and contaminants databases, and proteins of interest sequences using Proteome Discoverer (v.2.1.0.81).
Identification of the nCPEB4 isoform interactome by immunoprecipitation coupled to mass spectrometry
Overexpressed nCPEB4âGFP, nCPEB4Î4âGFP and GFP (control) were immunoprecipitated from differentiated N2a cells. Cells were lysed in ice-cold RIPA buffer containing 50âmM Tris HCl pHâ8, 1% Nonidet P-40 (NP40), 0.1% SDS, 1âmM EDTA, 150âmM NaCl, 1âmM MgCl2, 1à EDTA-free complete protease inhibitor cocktail (Roche, 5056489001) and phosphatase inhibitor cocktails (Sigma, P5726, and P0044). Cells were subsequently sonicated for 5âmin at low intensity with a standard bioruptor diagenode. Following centrifugation (4â°C for 10âmin at maximum speed), supernatants were collected, precleared and immunoprecipitated overnight at 4â°C with 50âμl GFP-conjugated Dynabeads proteinâA (Invitrogen). Beads had previously been conjugated with 10âμl anti-GFP antibody (Invitrogen, A6455) diluted in 500âμl PBS 1à for 2âh at room temperature. After immunoprecipitation, beads were washed with cold RIPA buffer (containing 0.05% NP-40 and 0.1% SDS) and eluted with Laemmli sample buffer. Eppendorf LoBind microcentrifuge tubes (Eppendorf, 30108116) were used for the entire protocol. The immunoprecipitated elutions were shortly run on 8% acrylamide 0.75âmm gels until the whole individual samples were compacted at the upper part of the running gel. Then, gels were stained with InstantBlue Coomassie for 1âh at room temperature. Bands corresponding to elutions were cut, washed and digested with 0.1âμgâμlâ1 trypsin, (Promega). Samples were digested with trypsin and digestion was stopped by the addition of 5% formic acid. Following evaporation, samples were reconstituted in 12âμl of 1% formic acid and 3% acetonitrile. In brief, mass spectrometry analysis of immunoprecipitates was performed as follows: samples were loaded into an Evotip trap column (Evosep) at a flow rate of 250ânlâminâ1. Peptides were separated using a EV1137 analytical column (Evosep) with a 88-min run and eluted with bufferâA (0.1% formic acid in H2O) and bufferâB (0.1% formic acid in acetonitrile). The column outlet was directly connected to an Easyspray (Thermo Scientific) fitted on an Orbitrap Eclipse Tribrid (Thermo Scientific). Spray voltage in the Easyspray source was set to 2.5âkV. The mass spectrometer was operated in data-dependent acquisition mode. The top speed (most intense) ions per scan were fragmented in the HCD cell and detected in the orbitrap. For peptide identification, searches were performed using Proteome Discoverer (v.2.5.0.400) software and run against databases including universal contaminants, mouse from Swissprot (2023/04) and bait proteins. Search parameters included trypsin enzyme specificity, allowing for two missed cleavage sites, oxidation in methionine, acetylation in the protein Nâterminus, methionine loss in the Nâterminus, and methionine loss in and acetylation in the Nâterminus as dynamic modifications, and carbamidomethyl in cysteine as a static modification. Protein hits in co-precipitates from each isoform were determined using a differential analysis of protein abundance in nCPEB4âGFP or nCPEB4Î4âGFP relative to GFP. Protein group abundance values from Proteome Discoverer were used for protein quantification, and cut-off values for the fold change (|FC|â>â1.5) and adjusted Pâvalue (padjâ<â0.05) were applied to define over-abundant significant proteins. Significant hits included proteins with no missing values in the three conditions (nCPEB4, nCPEB4Î4 or GFP) or in only one condition, for which value imputation was performed. Only significant hits were considered for subsequent analyses. Protein hits differentially represented in nCPEB4Î4âGFP versus nCPEB4âGFP were determined by a differential abundance analysis between the two baits, applying fold change (|FC|â>â1.5) and padjâ<â0.05) as cut-off values.
Competition experiments
Competition experiments were performed as previously described10 using 23ânl of 500ângâμlâ1 in vitro transcribed and polyadenylated RNAs encoding for HA-tagged xCPEB1 and xCPEB4 RRMs and variants. Not injected was considered as 0% competition whereas HA-xCPEB1 RRM was considered as 100% competition.
Plasmids for protein expression in Escherichia coli
An insert codifying for the nCPEB4(NTD) protein sequence (UniProt identifier Q17RY0-2, residues 1â448) was ordered in GenScript subcloned in a pET-30a(+) vector. The His6 tag and Sâtag from the plasmid were removed by PCR using a NEB Q5 site-directed mutagenesis kit, with oligonucleotides purchased from Sigma-Aldrich. The histidine to serine mutants were ordered from GenScript subcloned in a pET-30a(+) vector in the NdeI and XhoI restriction enzymes positions. The nCPEB4Î4, ÎHC and Î4ÎHC mutants were generated by PCR mutagenesis on the nCPEB4(NTD) plasmid using a NEB Q5 Site-directed mutagenesis kit, with oligonucleotides purchased from Sigma-Aldrich. The sequences of the N-terminal domain of nCPEB4 and mutants used for the in vitro experiments are described in Supplementary Methods.
Protein expression and purification for in vitro experiments
E.âcoli B834 cells were transformed with the pET-30a(+) plasmids. For non-isotopically labelled protein, the cells were grown in LB medium at 37â°C until the optical density at 600 nm (OD600) wasâ0.6, and then the cultures were induced with 1âmM IPTG for 3âh at 37â°C. For 15N or 15N,13C isotopically labelled protein, the cells were grown in LB medium until OD600â=â0.6 and then transferred into M9 medium53 (3âlitres LB for 1âlitre M9) containing [15N]H4Cl or [15N]H4Cl and [13C]glucose, respectively, and then the cultures were induced with 1âmM IPTG overnight at 37â°C. The cultures were then centrifuged for 30âmin at 4,000âr.p.m., and the cells were resuspended with lysis buffer (50âmM Tris-HCl, 1âmM DTT, 100âmM NaCl, 0.05% Triton X-100, at pHâ8.0, and supplemented with 500âμl of PIC and 500âμl of 100âmM PMSF).
The cells were lysed by sonication and centrifuged for 30âmin at 20,000âr.p.m. The pellet was washed first with wash-1 buffer (20âmM Tris-HCl, 1âmM DTT, 1âM NaCl, 0.05% Triton X-100, at pHâ8.0, and supplemented with 500âμl of PIC, 500âμl of 100âmM PMSF, and 50âμl of 5âmgâmlâ1 DNAse) and then with wash-2 buffer (20âmM Tris-HCl, 1âmM DTT, 0.1 M l-arginine, at pHâ8.0). The pellet was resuspended with the nickel-A buffer (25âmM Tris-HCl, 1âmM DTT, 50âmM NaCl, 8âM urea and 20âmM imidazole, at pHâ8.0) and centrifuged for 30âmin at 20,000âr.p.m. The supernatant was injected at room temperature into a nickel affinity column and eluted with a gradient from 0 to 100% of nickel-B buffer (25âmM Tris-HCl, 1âmM DTT, 50âmM NaCl, 8âM urea and 500âmM imidazole, at pHâ8.0). The fractions with protein were pooled, and 1âmM EDTA was added. The sample was injected into a size exclusion Superdex 200 16/600 (GE Healthcare) column, running at 4â°C in size exclusion buffer (25âmM Tris-HCl, 1âmM DTT, 50âmM NaCl and 2âM urea, at pHâ8.0). The fractions with protein were pooled and concentrated to approximately 150âμM. The sample was dialysed against the final buffer (20âmM sodium phosphate, 1âmM TCEP and 0.05% NaN3, at pHâ8.0), fast frozen in liquid nitrogen and stored at â80â°C.
Peptide for in vitro experiments
The me4(GS)3me4 synthetic peptide with amidated or Cy3-modified Câterminus and acetylated Nâterminus was obtained as lyophilized powder with >95% purity from GenScript. The peptide was dissolved in 6âM guanidine thiocyanate and incubated with agitation overnight at 25â°C. The sample was then centrifuged at 15,000âr.p.m. for 10âmin. The supernatant was extensively dialysed against the final buffer (20âmM sodium phosphate, 1âmM TCEP and 0.05% NaN3, at pHâ8.0)54. The peptide sample was then manipulated in the same way as the protein samples, as detailed below.
Sample preparation for in vitro experiments
All samples were prepared on ice as follows. First, a buffer stock solution consisting of 20âmM sodium phosphate buffer with 1âmM TCEP and 0.05% NaN3 was pH adjusted to 8.0 (unless otherwise indicated) and filtered using 0.22âμm sterile filters (buffer stock). A 1âM NaCl solution in the same buffer was also pH adjusted to 8.0 (unless otherwise indicated) and filtered (salt stock). The protein samples were then thawed from â80â°C on ice, pH adjusted to 8.0 (unless otherwise indicated) and centrifuged for 5âmin at 15,000âr.p.m. at 4â°C. The supernatant (protein stock) was transferred to a new Eppendorf tube, and the protein concentration was determined by measuring its absorbance at 280ânm. The samples were prepared by mixing the correct amounts of buffer stock, protein stock and salt stock, as well as other indicated additives in the experiments, to reach the desired final protein and NaCl concentrations.
Apparent absorbance measurement as a function of temperature
The absorbance of the samples was measured at 350ânm (A350ânm) using 1âcm pathlength cuvettes and a Cary100 ultravioletâvisible spectrophotometer equipped with a multicell thermoelectric temperature controller. The temperature was increased progressively at a ramp rate of 1â°Câminâ1. The cloud point (Tc) values were determined as the maximum of the first-order derivatives of the curves, and the absorbance increase (ÎA) represents the difference between the maximum and the minimum absorbance values of the samples during the temperature ramp.
For the experiment to quantify the reversibility of condensation, a 20âμM protein with 100âmM NaCl sample was prepared on ice. It was then split into 4 Eppendorf tubes and a temperature ramp was carried out with the first one after centrifugation for 2âmin at 5â°C and 15,000âr.p.m. Once the Tc and ÎA for condensation had been determined, the other 3 samples were heated 10â°C above the Tc for 2.5âmin and then cooled to 10â°C below the Tc for 5 more min. This procedure was repeated 1, 2, or 3 times for each sample. Next, the samples were centrifuged for 2âmin at 5â°C and 15,000âr.p.m., and a temperature ramp was carried out to determine their respective Tc and ÎA values (Extended Data Fig. 7d).
Microscopy in vitro
For microscopy imaging, 1.5âμl of sample was deposited in a sealed chamber comprising a slide and a coverslip sandwiching double-sided tape (3M 300 LSE high-temperature double-sided tape of 0.17âmm thickness). The coverslips used had been previously coated with PEG-silane following a published protocol55. The imaging was always performed on the surface of the coverslip, where the condensates had sedimented.
The DIC microscopy images were taken using an automated inverted Olympus IX81 microscope with a Ã60/1.42 oil Plan APo N or a Ã60/1.20 water UPlan SAPo objective using the Xcellence rt (v.1.2) software.
For fluorescence microscopy experiments, the purified proteins were labelled with DyLight 488 dye (DL488, Thermo Fisher Scientific) or Alexa Fluor 647 dye (AF647, Thermo Fisher Scientific). The labelling, as well as the calculation of the labelling percentage and the determination of the protein concentration, was performed following the providerâs instructions. The final samples contained 0.5âµM of labelled protein and/or peptide out of the total indicated concentrations.
FRAP experiments were recorded using a Zeiss LSM780 confocal microscope system with a Plan ApoChromat Ã63/1.4 oil objective. Condensates of similar size were selected, and the bleached region was 30% of their diameter. The intensity values were monitored for different ROIs: ROIâ1 (bleached area), ROIâ2 (entire condensate) and ROIâ3 (background signal). The data were fitted using EasyFrap software50 to extract the kinetic parameters of the experiment (recovery half-time and mobile fraction).
Super-resolution microscopy images of the multimers and their time evolution were taken at 25â°C in a Zeiss Elyra PS1 LSM 880 confocal microscope using the Fast Airyscan mode with an alpha Plan ApoChromat Ã100/1.46 oil objective. The pixel size was kept constant at 40ânm.
Fluorescence microscopy images of the condensates and aggregates were taken at 37â°C in a Zeiss Elyra PS1 LSM 880 confocal microscope with an Airyscan detector using a Plan ApoChromat Ã63/1.4 oil objective. The quantification of the aggregation process was done by image analysis using Fiji/ImageJ. The regions with the fluorescence signal not stemming from the background or the spherical condensates were selected. The percentage of the area of the field of view occupied by this selection corresponds to the aggregation value of the sample. The partitioning of the proteins in the condensates was calculated by image analysis using Fiji/ImageJ. The partitioning for each condensate was calculated by dividing the mean intensity of the condensate by the mean intensity of a ring of 1âμm thickness around the condensate.
RNA for in vitro experiments
The RNA used for in vitro experiments is a fragment of the 3â²âUTR of cyclin B1 mRNA from X.âlaevis containing only one CPE site56,57, 5â²-AGUGUACAGUGUUUUUAAUAGUAUGUUG-3â². We used it as a control to study whether it influences the properties of the condensates. RNA caused a slight decrease in the phase-separation propensity of both isoforms, larger for nCPEB4(NTD) than for nCPEB4Î4(NTD), which we attribute to interactions with positively charged amino acids involved in the intermolecular interactions driving condensation (Extended Data Fig. 5f). Notably, however, the presence of RNA in the samples did not alter their propensity to aggregate (Extended Data Fig. 5g).
Saturation concentration measurements
Saturation concentration measurements of nCPEB4(NTD) and the histidine to serine mutants were carried out by incubating the samples at 40â°C for 5âmin, followed by centrifugation at 5,000âr.p.m. for 1.5âmin at 40â°C. The concentration of protein in the supernatant (csat) was determined by absorbance measurement at 280ânm.
NMR spectroscopy
The samples were prepared as indicated in the section âSample preparation for in vitro experimentsâ using isotopically labelled protein (15N- or 15N,13C-labelled). The prepared final samples were again pH adjusted to the desired value immediately before measurement. All the measurements were acquired at 5â°C using 3âmm NMR tubes with a sample volume of 200âµl.
All NMR experiments, except the diffusion measurements, were carried out on a Bruker Avance NEO 800âMHz spectrometer equipped with a TCI cryoprobe. All NMR samples contained 100âμM protein concentration (unless otherwise indicated) in 20âmM sodium phosphate buffer with 1âmM TCEP, 0.05% NaN3, 7% D2O and 2.5âμM DSS for referencing, at pHâ8.0 (unless otherwise indicated). Samples with denaturant agent contained the indicated concentrations of d4-urea.
A 15N,13C-labelled sample at 280âμM for nCPEB4(NTD) or 200âμM for nCPEB4Î4(NTD) with 4âM urea at pHâ7.0 was used for backbone resonance assignment. A series of nonlinear sampled 3D triple resonance experiments were recorded, including the BEST-TROSY version58 of 1HN-detected HNCO, HN(CA)CO, HNCA, HN(CO)CA, HNCACB, HN(CO)CACB and (H)N(CA)NH. Also, additional 1Hα-detected HA(CA)CON and (HCA)CON(CA)H experiments59 were measured for nCPEB4(NTD). Backbone resonance assignments were performed using CcpNmr60 (v.2.4.2). The NMR assignments are available from the Biological Magnetic Resonance Data Bank (identifiers 51875 and 52346 for nCPEB4(NTD) and nCPEB4Î4(NTD), respectively).
pH titrations from 7.0 to 8.0 were carried out to transfer NH assignments to the final experimental conditions. Standard 2D 1H,15N-HSQC or BEST-TROSY experiments were measured at 7.0ââ¤âpHââ¤â7.25. 2D 1H,15N-CP-HISQC61 experiments were used at pHââ¥â7.5 to reduce the effects of chemical exchange with water. For the urea titrations from 0 to 4âM at pHâ8.0, 1H,15N-CP-HISQC experiments were measured. In the 1H,15N-CP-HISQC for the detection of arginine side-chain resonances, the 15N carrier was placed at 85âppm, and 13C pulses for decoupling were centred at the chemical shift of 13Cδ (42âppm) and 13C⥠(158âppm) arginine side chains.
Standard 2D 1H,13C-HSQC experiments of 500âμM 15N,13C-labelled nCPEB4(NTD) were measured in the absence and presence of 4âM urea to monitor specific amino acid side chains easily identifiable by their typical 1H and 13C random coil chemical shifts.
For histidine pKa determination, 2D 1H,13C-HSQC spectra of 75âμM 15N,13C-labelled nCPEB4(NTD) were measured in the presence of 4âM urea at pH values between 5.58 and 8.31. The pH-induced changes of the chemical shifts of histidine side chains (1H and 13C, both aliphatic and aromatic) were fitted to a sigmoid function to obtain an apparent pKa for all these histidine resonances in nCPEB4(NTD).
Non-uniform sampled experiments were processed using qMDD62 (v.3.2). 2D 15N-correlations (1H,15N-HSQC, 1H,15N-CP-HISQC, BEST-TROSY) and 2D 1H,13C-HSQC were processed using NMRPipe63 and Topspin (v.4.0.8) (Bruker), respectively.
15N-edited X-STE diffusion experiments64 of 100âμM 15N-labelled nCPEB4(NTD) in the absence and presence of 2âM urea were performed on a Bruker Avance III 600âMHz spectrometer equipped with a TCI cryoprobe. An encoding/decoding gradient length of 4.8âms and a diffusion delay of 320âms were used. The hydrodynamic diameter of nCPEB4(NTD) was estimated using dioxane as a reference molecule. Diffusion measurements under identical experimental conditions were carried out for dioxane, using in this case the PG-SLED sequence65. A gradient time (δ) of 1.6âms and a diffusion time (Î) of 70âms were used. Diffusion coefficients were obtained by fitting the data to a mono-exponential equation using MestreNova (v.14.2.1-27684).
Dynamic light scattering
Dynamic light scattering (DLS) measurements were taken with a Zetasizer Nano-S instrument (Malvern) equipped with a He-Ne of 633ânm wavelength laser. Immediately before the measurement, the prepared samples were centrifuged for 5âmin at 15,000âr.p.m. at 4â°C, and only the supernatant was subjected to measurement. Three measurements were performed for each sample, each of the measurements consisting of 10 runs of 10âs each. The experiments were carried out at 5â°C unless otherwise indicated. The deconvoluted hydrodynamic diameters arise from the mean of the peak of the intensity deconvolution of the data.
Size exclusion chromatography coupled to multi-angle light scattering
Size exclusion chromatography coupled to multiangle light scattering (SECâMALS) experiments were performed by loading a 160âμM nCPEB4(NTD) with 0âmM NaCl sample into a Superose 6 increase 10/300 GL column (GE Healthcare) mounted on a Shimadzu Prominence Modular HPLC with a SPD-20 UV detector (Shimadzu) coupled to a Dawn Heleos-II multi-angle light scattering detector (18 angles, 658ânm laser beam) with an Optilab T-rEX refractometer (Wyatt Technology). The SEC-UV/MALS/RI system was equilibrated at 25â°C with 20âmM sodium phosphate buffer with 1âmM TCEP and 0.05% NaN3 at pHâ8.0. Data acquisition and processing were performed using Astra 6.1 software (Wyatt Technology).
Liquid-phase transmission electron microscopy
Transmission electron microscopy (TEM) experiments were performed on a 50âμM nCPEB4Î4(NTD) sample with 0âmM NaCl. The sample was first imaged in solid-state TEM using the same set-up as described below for liquid-phase TEM (LPEM). Pre-screening samples in solid-state TEM before the LPEM imaging procedure is routinely done to pre-screen the structures that will be imaged in liquid later. The sample with no stain was deposited onto 400âmesh Cu grids, which were plasma discharged for 45âs to render them hydrophilic and to allow optimal sample wettability.
LPEM imaging was performed using a JEOL JEM-2200FS TEM microscope. The system was equipped with a field emission gun operating at 200âkV and an in-column Omega filter. The images were acquired with a direct detection device, the in-situ K2-IS camera (Gatan). The Ocean liquid holder, from DENSsolutions, was used to image the structure and dynamics of the specimens. The liquid samples were encased into two silicon nitride (SiXNy) chips. These chips had a 50-nm-thick SiXNy electron-transparent window of dimensions 10âÃâ200âμm. One of these chips had a 200ânm spacer that acts as a pillar and defines the liquid cell thickness, that is, zâheight, and hence the liquid thickness in the experiments. The chips were cleaned in HPLC-graded acetone followed by isopropanol for 5âmin each to remove their protective layer made of poly(methyl methacrylate). Afterwards, the chips were plasma-cleaned for 13âmin to increase their hydrophilicity. Next, 1.5âμl of non-stained sample was deposited on the previously prepared 200-nm-spacer chip. The drop-casted sample was enclosed by the spacer-less chip, thus sealing the liquid chamber. Then, 300âμl of the sample solution was flushed in the liquid holder at 20âμlâminâ1 with a syringe pump to ensure that the liquid cell inlet and outlet pipes were filled with the solution. We waited 5âmin after collecting the sample solution from the outlet tube to minimize the convection effects from the flowing process. The liquid holder was introduced in the microscope, where imaging was performed in TEM mode and static conditions, that is, not in flow. To limit the electron beam dose (20âeââà â2) on the specimen, images were collected at the minimum spot size (numberâ5) with a small condenser lens aperture (CLA numberâ3). For our investigations, dose fractionation videos were recorded in counted mode at 20 frames per s with the K2 camera. Every image was recorded in the format of 4-byte greyscale and required the full size of the detector.
The images and videos recorded were corrupted by noise, which significantly reduced the quality of the images, complicating any further analysis. Therefore, the Noise2Void (N2V) machine learning-based approach was adopted to overcome this problem66. Unlike the conventional machine learning-based approach, N2V reduces the noise of an image without any need for the corresponding noiseless image. This requirement makes the N2V ideal to process LPEM images, as noiseless images in liquid TEM are impossible to record. However, N2V requires an extensive dataset (also known as a training set) to fulfil its task, a common requirement to machine-learning-based approaches. Conventionally, the training set has to contain thousands of hundreds of images recorded with the same imaging settings to produce high-quality estimation of the noise distribution. Unfortunately, in our case, only single videos (image sequence) recorded at different imaging conditions were available. Therefore, the training set was created by sampling the image sequence every two frames to not bias the training process of the N2V. The remaining half of the image sequence was processed and used to derive the presented results.
To train the N2V, 3,050 frames were selected, extracting 128 different non-overlapping squared patches (that is, portions of the pixels of the image) of 64âÃâ64 pixels from each of them. Moreover, the N2V was iterated for 100 epochs, a trade-off value between performances and processing time. The training was performed by extracting 128 random patches (64âÃâ64 pixels) from each training image. These values produced the best results in a short processing time.
Molecular simulations
Molecular dynamics simulations were performed using the single-bead-per-residue model CALVADOS (v.2)22,23 implemented in OpenMM (v.7.5)67. All simulations were conducted in the NVT ensemble at 20â°C using a Langevin integrator with a time step of 10âfs and friction coefficient of 0.01âpsâ1. In our simulations, pH was modelled through its effect on the charge of the histidine residues (qHis)68.
Direct coexistence simulations were performed with 100 chains in a cuboidal box of side lengths [Lx, Ly, Lz]â=â[25, 25, 300]ânm. Simulations were run in nâ=â3 independent replicas for at least 55âµs each. The systems readily formed a protein-rich slab spanning the periodic boundaries along the x and yâcoordinates. The initial 2âµs of the simulation trajectories were discarded, and time-averaged concentration profiles along the zâaxis were calculated after centring the condensates in the box as previously described22.
To model protein multimers, 400 chains were initially placed at random positions in a cubic box of side length 188ânm and simulations were run in nâ=â2 replicas for 16âµs each. The formation and dissolution of protein multimers was monitored using a cluster analysis implemented in OVITO69. Proteins were clustered on the basis of the distances between their centres of mass, using a cut-off of 1.5 times the average radius of gyration of the protein. Contact maps were calculated between a chain in the middle of the condensate or multimer and the surrounding chains. Contacts were quantified using the switching function c(r)â=â0.5âââ0.5âÃâtanh[(râââÏ0)/r0], where r is the intermolecular distance between two residues, Ï0â=â1ânm, and r0â=â0.3ânm.
To match the conditions of the experiments with which the simulation results are compared, direct coexistence and multimer simulations were performed at ionic strengths of 150âmM and 60âmM, respectively. Configurations were saved every 2âns for slabs and every 5âns for multimer simulations.
Simulation trajectories were analysed using MDTraj70 (v.1.9.6) and MDAnalysis71,72 (v.1.1). The molecular visualizations presented in Fig. 2j were generated using VMD73 (v.1.9.4).
Characterization of the multimers
To investigate which specific residues of nCPEB4(NTD) drive its condensation, we used solution NMR spectroscopy, a technique that can provide residue-specific information on the conformation and interactions of intrinsically disordered proteins74. Indeed, when conditions are insufficient for condensation, the same interactions driving this process can cause the monomer to collapse into a state that can be characterized at residue resolution, thus providing this key information75. Under such conditions (Supplementary Fig. 5a), nCPEB4(NTD) had a spectrum characteristic of a collapsed intrinsically disordered protein, in which only 13% of the NMR signals were visible (Supplementary Fig. 5b, in light green). We analysed the sample by DLS to confirm its monomeric nature, but we detected only nCPEB4(NTD) multimers with a hydrodynamic diameter of approximately 55ânm, which is much larger than that predicted for a monomer76 (approximately 11ânm) (Supplementary Fig. 5c).
To characterize the multimers, also known as clusters, which have also been observed for phase-separating proteins with intrinsically disordered domains77,78,79,80, we first determined whether their formation is reversible. To this end, we performed DLS analysis of increasingly diluted samples of nCPEB4(NTD) under the same solution conditions. We observed that at 0.5âµM, the multimers seemed to be in equilibrium with a species with a hydrodynamic diameter close to that predicted for the monomer (Extended Data Fig. 3e, in green). Next, at this concentration, we modified solution conditions to favour condensation by increasing the temperature and ionic strength, as well as by decreasing the pH. In all cases, we observed a decrease in the signal corresponding to the monomer and an increase in that corresponding to multimers, thus indicating a shift of the multimerization equilibrium (Extended Data Fig. 3e). This result suggests that the nCPEB4(NTD) multimers are stabilized by the same types of interactions as the condensates (Extended Data Fig. 1m and Fig. 2e,k).
These findings prompted us to study whether these multimers grow over time to become condensates observable by optical and fluorescence microscopy, that is, whether they represent intermediates in the condensation pathway. To this end, we first analysed, over 15âh, samples of freshly prepared nCPEB4(NTD) multimers by DLS. We observed a progressive increase in size and polydispersity up to an average hydrodynamic diameter of approximately 90ânm and a polydispersity index of approximately 0.10 (Supplementary Fig. 5d). We also determined the morphology of the multimers at the nanoscale by LPEM, confirming that they are spherical and have a diameter of 30â50ânm (Extended Data Fig. 3f and Supplementary Fig. 5e). Finally, super-resolution microscopy confirmed that the multimers grow with time, thus becoming larger spherical particles resembling condensates (Extended Data Fig. 3g). It is possible that the nCPEB4 multimers here identified correspond to mesoscopic condensates, equivalent to those observed by optical microscopy, albeit smaller; however, as their dimensions preclude their thorough physical characterization, we consider it appropriate to consider them distinct species in this work.
We next carried out experiments to determine the nature of the species giving rise to the residue-specific NMR signals observed in the presence of multimers. To this end, we analysed a sample of multimers by SECâMALS. This approach showed that the multimers are assemblies of approximately 350 nCPEB4(NTD) molecules (Supplementary Fig. 5f), corresponding to a molecular weight of approximately 1.7âÃâ107âDa. Next, we performed 15N-edited X-STE experiments to measure the diffusion coefficient of the species giving rise to the signals detected by NMR, from which we derived that they diffuse much more slowly than a monomer, with a value in semi-quantitative agreement with that obtained by DLS and LPEM (Supplementary Fig. 5g), thereby indicating that they correspond mainly to multimers. We concluded that solution NMR can be used to identify the most flexible regions of the nCPEB4(NTD) sequence under conditions in which it forms multimers, presumably corresponding to those least involved in the interactions stabilizing them81,82,83.
To assign the NMR signals to specific residues, we progressively added urea and observed that at 1.5â2âM, the urea concentration necessary to dissociate the multimers, the signals of most residues could be detected (Extended Data Fig. 3h,i and Supplementary Fig. 5b) and stemmed from a species with the diffusion coefficient expected for a nCPEB4(NTD) monomer (Supplementary Fig. 5g). A comparison of the spectra obtained in the absence and presence of denaturant revealed that the signals observed for the multimer corresponded to residues between positions approximately 50 and 150 in the sequence of nCPEB4(NTD): this region of sequence is devoid of aromatic and positively charged residues and instead rich in negatively charged ones (Extended Data Fig. 3h,j). Despite the presence of 4âM urea, the spectrum of monomeric nCPEB4(NTD) had a wide range of intensities. An analysis of signal intensity as a function of residue type revealed particularly low values for histidine and arginine (Extended Data Fig. 3k), which suggested that these residue types are involved in transient interactions and explain the very low signal intensity of the histidine-rich HClust (residues 229â252) and the arginine-rich me4 (residues 403â410) (Extended Data Fig. 3h).
To facilitate the interpretation of these results, we monitored the formation of nCPEB4(NTD) multimers in molecular simulations performed using the CALVADOS model22,23. Contacts calculated between a chain in the centre of the multimer and the surrounding chains in assemblies of 130â170 chains showed that the C-terminal region, including HClust and me4, is more involved in intermolecular interactions than the N-terminal region rich in aspartate and glutamate residues (positions 72â147) (Extended Data Fig. 3l). The simulations therefore support the conclusion that the decreased NMR signal intensity in the C-terminal region reflects an increased number of contacts of these residues within the condensates. Finally, to further characterize the interactions stabilizing the multimers, we performed 1H,13C-HSQC experiments to analyse the intensity of side-chain signals without urea, when nCPEB4(NTD) is multimeric, and in 4âM urea, when nCPEB4(NTD) is instead monomeric (Extended Data Fig. 3m and Supplementary Fig. 5hâj). We observed that the intensities, especially those of the signals corresponding to histidine, tryptophan and arginine residues, are lower for the former than for the latter. We also performed 1H,15N-CP-HISQC experiments to study the arginine side-chain NH signals in the same samples and found that they are undetectable in the absence of the denaturant but can be observed in its presence (Supplementary Fig. 5k). Taken together, our results indicate that the multimers formed by nCPEB4(NTD) on the condensation pathway are stabilized mainly by interactions involving histidine and arginine residues, which are predominantly located in the C-terminal region (Supplementary Fig. 5l).
Protein sequence analysis
Cprofiler (http://www.cprofiler.org/) was used to determine the enrichment score of each amino acid type in the protein sequence compared with the DisProt3.4 database84,85. The protein sequences analysed correspond to residues 1â448 for nCPEB4(NTD) (UniProt identifier Q17RY0-2), residues 1â311 for CPEB2(NTD) (UniProt identifier Q7Z5Q1-3) and residues 1â452 for CPEB3(NTD) (UniProt identifier Q7TN99-1).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.