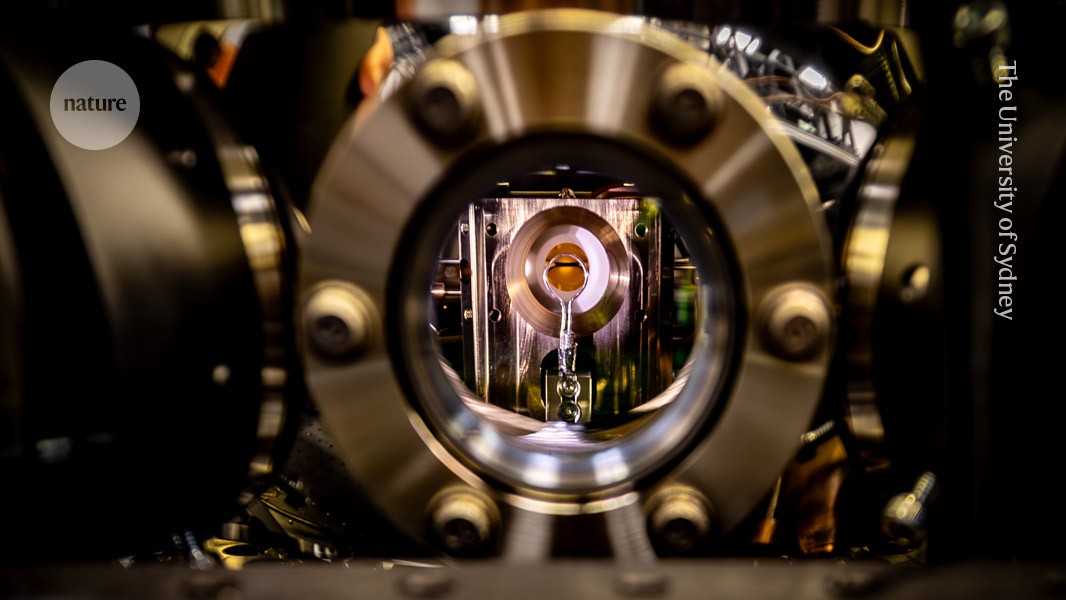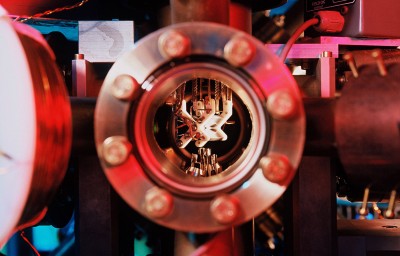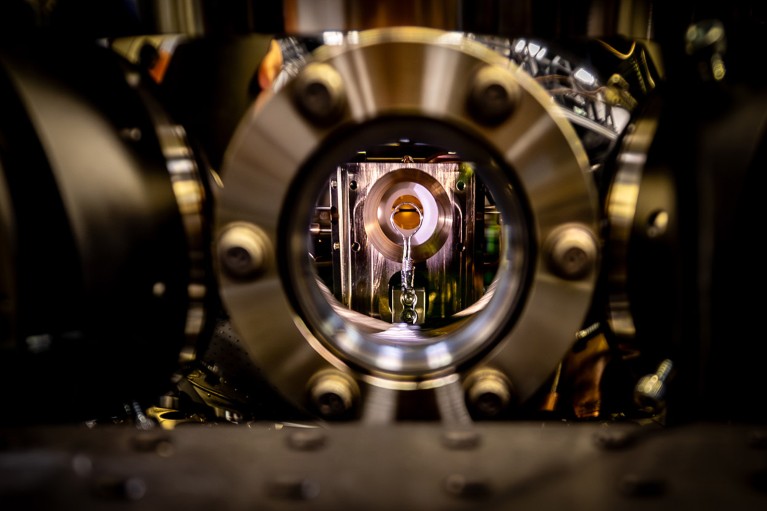
Inside the trapped-ion quantum computer that carried out the chemistry simulation.Credit: The University of Sydney
A single atom has performed the first full quantum simulations of how certain molecules react to light. The researchers who carried out the feat say that their minimalistic approach could dramatically speed the path towards a ‘quantum advantage’ — when quantum computers will be able to predict the behaviour of chemicals or materials in ways that are beyond the reach of ordinary computers.
“The key advantage of this approach is that it is incredibly hardware-efficient,” says Ting Rei Tan, an experimental quantum physicist at the University of Sydney. The single atom can encode the information that is normally spread across a dozen or so ‘qubits’, the computational units used in most quantum computers. The findings were published on 14 May in the Journal of the American Chemical Society1.
No quantum computer had simulated this level of complexity in the energy levels of molecules before, says Alán Aspuru-Guzik, a computational chemist at the University of Toronto in Canada. “This is a tour-de-force that will remain in the history books.”
Excited electrons
Tan and his colleagues simulated the behaviour of three different organic molecules, allene, butatriene and pyrazine, when they are hit with an energetic particle called a photon. When this happens, it triggers a cascade of events in the molecule that affects both how its atoms move with respect to each other — vibrating like balls connected by springs — and how its electrons jump to higher-energy, or excited, states. Understanding the precise sequence of these events can help chemists to design molecules that channel energy in the most useful or efficient way, for example in solar panels or in sunscreen lotion.
Meet ‘qudits’: more complex cousins of qubits boost quantum computing
The researchers found a way to encode these different parameters into a single ytterbium ion trapped in a vacuum using pulsating electric fields: the excitations of the molecule’s electrons corresponded to similar excitations in one of the ion’s electrons, and two different vibrational modes were represented by the ion wiggling inside its trap in two different directions. The team also nudged the ion with laser pulses to tailor how all of the states interacted with one another. This forced the ion to evolve over time, meaning it could mimic how the corresponding molecules act after being hit by a photon.