Matrix preparation
Collagen extraction
Type I collagen monomers were extracted from rat tail tendons following a classical procedure. Fresh tendons were washed with phosphate-buffered saline solution and solubilized in 0.5âM acetic acid. The raw solution was centrifuged and the supernatant was selectively precipitated with 0.7âM NaCl. Precipitated type I collagen was solubilized in 0.5âM acetic acid and then desalted by dialysis against 0.5âM acetic acid. The concentrations of type I collagen solutions were assessed by hydroxyproline titration and adjusted to a final stock concentration of approximately 3âmgâmlâ1.
Matrix synthesis
All of the experiments were carried out at room temperature (19â±â2â°C) to prevent collagen denaturation and sterility was kept throughout the procedure. The matrices were stored in distilled water at 4â°C before implantation.
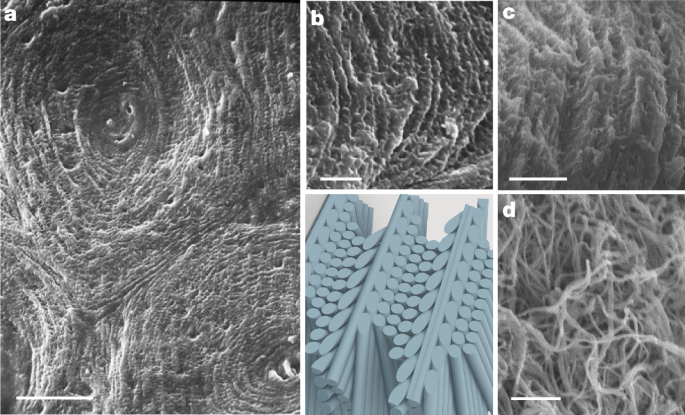
Col40 (rat model)
The collagen solution was progressively concentrated up to 40âmgâmlâ1 by slow evaporation of the solvent in a safety cabinet52. Then, fibrillogenesis in vitro was performed under ammonia vapours for 24âh. This step allows the stabilization of the liquid-crystalline organization into dense fibrillar matrices53. Finally, matrices were punched with an 8-mm steel trepan and rinsed with sterile phosphate-buffered saline until neutralization of the pH.
Col100 (rat model)
Below the critical concentration of 40âmgâmlâ1, type I collagen molecules do not organize52. However, above this threshold concentration, they undergo a spontaneous transition into ordered liquid-crystalline phases owing to the lyotropic properties of acidic type I collagen solution in vitro19. After a solâgel transition leading to collagen fibril formation, the geometries of fibrillary networks closely mimic those found in living tissues, depending on the targeted concentration (at least 80âmgâmlâ1 for cholesteric bone-like mesophase)53,54. The Col100 matrix was prepared using a procedure that combines injection and reverse-dialysis processes to increase the collagen concentration17. A 15âml volume of about 1âmgâmlâ1 soluble acidic collagen solution (0.5âmM acetic acid) was continually injected (rate range 1âμlâminâ1 to 15âmlâminâ1) in a closed dialysis chamber (3âmm thickness and 10âmm width) for 8âdays. The bottom of the chamber contained a dialysis membrane with a molecular weight cut-off of 12â14âkDa. The reverse-dialysis process was set against polyethylene glycol (35âkDa, Fluka) dissolved in 0.5âM acetic acid up to about 150âmgâmlâ1. The flow of the collagen solution was controlled to maintain the same pressure on each side of the dialysis membrane. After injection, dialysis was continued for 8âdays, to obtain a homogeneous concentration in the samples. The pH was then increased to a range of 9â10 by ammonia gas diffusion for 4âdays to induce collagen fibrillogenesis and stabilize the liquid-crystalline organization into dense fibrillar matrices29. The matrices were then removed from the dialysis chamber and washed several times in double-distilled water until neutralization. Finally, matrices were punched with an 8-mm steel trepan before implantation.
Col (ewe model)
The procedure matched the Col100 matrix except that: (1) the initial volume of the acid solution was 30âml and (2) the mould dimensions were diameter 8âmm and depth 13âmm. Here the process to elaborate the materials ends with the removal of the stabilized fibrillar matrix directly from the mould that allows the shaping of the collagen construct.
Col-CHA (ewe model)
The Col-CHA matrix was prepared as follows: a volume of a 3âmgâmlâ1 soluble acidic collagen solution (0.5âmM acetic acid) was mixed with a CaCl2/NaH2PO4/NaHCO3 acidic solution (0.5âmM acetic acid). According to a previously described procedure (col/CHA in ref.â20), the procedure is consistent with the synthesis of preferentially B-type CHA, which has a formula of Ca10âx(PO4)6âx(CO3)x(OH)2âx with 0ââ¤âxââ¤â2. The pH was adjusted to 2.2. The final concentration of the collagen solution was about 1âmgâmlâ1 and the final ionic strength was 165.9âmM. The procedure matched the Col matrix except that the initial volume of the acid solution was 30âml.
ColCG-CHA (ewe model)
The procedure matched the Col-CHA matrix except that commercial acidic clinical-grade collagen was used instead of collagen extracted from rat tail tendons to avoid inflammatory response. Bovine skin type I collagen diluted in acetic acid at a concentration of 5âmgâmlâ1 was purchased from Symatese (reference ACI070). The use of clinical-grade collagen was chosen to eliminate any potential immunologic responses arising from variations in collagen-purification processes55, thereby enabling the evaluation of our samples as potential bone biomaterials. Although both collagen solutions exhibit lyotropic properties and the ability to form fibrils necessary to produce high-density twisted plywood collagen matrices, clinical-grade collagen demonstrates higher purity and greater thermal stability (see Extended Data Fig. 7).
Commercially available mineral-based substitutes
Vitoss 1.2âcc blocks (reference 2102-0013 Stryker) is composed of a porous (up to 90%) structure of β-tricalcium phosphate. The Vitoss block was shaped with a scalpel to fit the defect. Mastergraft 5cc Granules (reference 7600105, Medtronic) is composed of 80% porous resorbable ceramic granules (15% HA, 85% β-tricalcium phosphate).
Autologous bone
AB fragments were extracted from iliac crest. The animalâs own blood serum was kept and the fragments were subsequently used as bone filler.
Reference human bone sample for SEM and TEM
The bone samples observed by SEM in Fig. 1a,b and by TEM in Fig. 3c were prepared as part of a previous study56.
Bone powder
The organic matrix (collagen and other proteins) was removed from bone sample by immersion in dilute NaClO aqueous solution treatment to extract the mineral particles (Extended Data Fig. 1), as previously described36.
Matrix characterization
SEM and elemental characterizations
Collagen matrices were fixed in 2.5% glutaraldehyde in a cacodylate solution (0.05âM). After washing in a cacodylate/saccharose buffer solution (0.05âM/0.6âM, pHâ7.4), dehydration in increasing ethanol baths, matrices were dried at the carbon dioxide critical point using a Leica EM CPD300. Samples coated with a 10ânm gold layer were observed in a Hitachi S-3400N at an accelerating voltage of 10âkV.
TEM
Matrices were fixed in glutaraldehyde, washed and dehydrated as described for SEM and embedded in araldite. The matrices prepared without mineral (Col40, Col100 and Col) were also post-fixed with 2% osmium tetroxide for 1âh at 4â°C before dehydration. Ultrathin sections (70ânm) were obtained, stained with uranyl acetate and observed in a FEI Tecnai G2 Spirit TWIN electron microscope operating at 120âkV.
WAXD (transmission mode)
Small parts of the mineral matrices were cut out of the bulk sample and inserted in X-ray cylindrical borosilicate capillary tubes. The tubes were flame-sealed to keep the samples hydrated and then placed directly in the vacuum chamber beam. X-ray diffraction experiments were performed with a S-MAX 3000 RIGAKU using a monochromatic CuKα radiation. The data were collected in the 5â60° range (2θ). The sample-to-detection distance was 0.059âm. The data were analysed using FIT2D (version 18, beta) software. The 2D WAXD patterns were collected with imaging plates and then scanned with 50âμm resolution. The diameter of the cylindrical beam dimension at the specimen was 400âμm and the sample thickness was approximately 1âmm.
Differential scanning calorimetry
Experiments were performed on a TA Q20 apparatus. The heating rate was set at 5â°Câminâ1 and the temperature ranged from 25â°C to 55â°C. About 20âmg of sample was weighed and put in an aluminium pan, the reference being an empty sealed aluminium pan. Collagen purity is determined on the basis of thermal stability. Collagen solutions typically exhibit an endothermic peak around 40â°C, indicating denaturation into gelatine that occurs through the irreversible unfolding of the triple helix57.
Polarized light microscopy
The materials were placed, without any treatment, between a glass slide and a coverslip. Observations were made using a transmission Zeiss Axio Imager A2 POL. The microscope was equipped with the standard accessories for examination of birefringent samples under polarized light (that is, crossed polars) and an Axiocam CCD camera.
SDS-PAGE
Sodium dodecyl sulfateâpolyacrylamide gel electrophoresis (SDS-PAGE) of type I collagen extracted from rat tail tendons and bovine dermis (clinical grade) was performed, using an acrylamide (10%)/bis-acrylamide (30%) gel in the presence of tris-HCl 1.5âM (pH 8.8), SDS, EDTA, glycerol and 2-mercaptoethanol. Migration was checked by adding bromophenol blue to the samples (0.05%). The separated protein bands were identified by comparison with a standard molecular mixture marker (Sigma-Aldrich) including the α chain of type I collagen (125.103âKDa).
Mechanical characterization
The local Youngâs modulus E of the Col40, Col100 and Col-CHA matrices was estimated at room temperature (22â°C) through microindentation (Piuma Nanoindenter, Optics 11 Life). To this aim, the Col40 and Col100 matrices were used as is (see Fig. 2c), whereas the Col-CHA matrix shown in Fig. 3e was cut into a piece of thickness about 1âmm. These matrices were immersed in their conservation medium at the bottom of a small Petri dish and their upper surface was indented using a spherical glass probe of radius R attached to a cantilever of calibrated stiffness k, with Râ=â105âµm and kâ=â0.45âNâmâ1 for Col40 and Râ=â51âµm and kâ=â0.26âNâmâ1 for Col100 and Col-CHA. Depending on the sample, these indentation tests involved forces ranging from 0.15âµN to 1.2âµN and penetration depths of 0.5â12âµm at the end of the loading phase, and to contact radii of 5â30âµm between the probe and the matrix. For each sample, about 200 independent indentations spaced by 50â200âµm were performed and each forceâdepth curve was fitted by a Hertzian contact model, yielding the effective Youngâs modulus Eeff at the locus of the indent. The local Youngâs modulus E was then computed through Eâ=âEeff(1âââν2), assuming a Poisson ratio ν of 0.5 for all samples. The statistical analysis of the Youngâs modulus data was performed using MATLAB (release R2018b) software.
Implantations
Rat calvaria critical-size defect
The study was reviewed and approved by the Animal Care Committee of the University Paris Descartes (no. P2.JLS.174.10) The 30 8-week-old (220â240âg) male Wistar rats used in the study were housed in individual cages at stable conditions in the animal facility of the Laboratoire Pathologies, Imagerie et Biothérapies Orofaciales, Université Paris Cité. The surgical procedures were performed as described previously24,25,58. After anaesthesia with an intraperitoneal injection of 100âmg per kg body weight of ketamine and 10âmg per kg body weight of xylazine hydrochloride (Centravet Alfort), the cranial area was shaved, disinfected and the skin was incised in the sagittal direction. The periosteum was then incised in the same direction and elevated to expose the underlying calvaria. An 8-mm defect was created in the centre of the calvaria using a steel trephine mounted on a low-speed dental handpiece, under sterile saline irrigation. The defects were randomly filled with dense collagen matrices, either twisted plywood organized fibrils (100âmgâmlâ1; Col100, nâ=â10) or randomly dispersed fibrils (40âmgâmlâ1; Col40, nâ=â10) or left empty (controls nâ=â8). The skin flap was then replaced and secured with interrupted sutures. After 10âweeks, rats were anaesthetized as previously and euthanized using exsanguination. The skulls were removed and fixed in 70% ethanol. Three animals were excluded from further analysis: in the control group, one animal died and another had an incomplete defect created during the surgical procedure; in the Col100 group, one animal had its underlying dura mater and mid-sagittal sinus damaged, thus introducing a technical bias.
Radiomorphometric analysis (rat)
The skulls were radiographed (exposure 13âkV, 12âmA, 15âmin) in a microradiography unit (model Sigma 2060, CGR) with X-ray film (Kodak Professional Films). Morphometry was performed at a constant magnification with a semi-automatic image analyser coupling the microscope to a video camera and a computer. The percentage of bone-defect closure, corresponding to the percentage of radiopacity in skull defect, was calculated as the following ratio: area of radiopaque formation in the defect/area of the defect created by trephinationâÃâ100 using ImageJ software (v1.52a).
µCT
µCT was performed in IMOSAR, Laboratoire de Biologie, Bioingénierie et Bioimagerie Ostéo-articulaires (B3OA), UMR CNRS 7052 INSERM U1271, Université de Paris. Representative samples were scanned using a desktop micro-X-ray computed tomography (Micro-CT SkyScan 1172, Bruker), source voltage 59âkV, source current 100âμA, image pixel size 17.77âμm. Each sample was rotated 180° with a rotation step of 0.7°, exposure time 90âms. 3D reconstruction and analysis were made with NRecon SkyScan software 1.7.1.6 (Bruker).
Statistical analysis
Given the high variability of the in vivo response and the related small sample size, the KruskalâWallis test (αâ=â0.05, no correction, multiple comparison using the MannâWhitney test) was used to assess overall differences between groups owing to its robustness in handling nonparametric data, whereas independent two-sided pairwise comparisons using the MannâWhitney U test were performed to detect any potential trends or variations between specific groups. The results were discussed following two levels of significance. Differences were considered strictly significant if Pâ<â0.05, whereas a Pâ<â0.10 indicates a trend in the data. The statistical tests conducted in this study are indicated in the figure legends as follows: *Pâ<â0.05 and **Pâ<â0.10. Data are given as meanâ±âs.d.
Histomorphometric analysis (rat)
After dehydration, the explants were embedded without demineralization in methyl methacrylate (Merck). 4-μm-thick sections were cut in the frontal plane with a Polycut E microtome (LEICA) in the central part of the defect. Sections were stained with toluidine blue, von Kossa or processed for enzyme histochemistry (Extended Data). Morphometry was performed at a constant magnification with a semi-automatic image analyser coupling the microscope to a video camera and a computer. TRAP sections were used to count the number of osteoclasts by millimetre based on 1/10th of the section. The von Kossa staining was used to quantify the percentage of mineralized tissue in the defect, calculated as the following ratio: area of matrix-related mineralized tissue/area of the defectâÃâ100.
Enzyme histochemistry (rat)
ALP activity was revealed with naphthol AS-TR phosphate (Sigma-Aldrich) and diazoted Fast Blue RR (Sigma-Aldrich). TRAP was detected using Fast Red TR Salt (Sigma-Aldrich) and naphthol AS-TR phosphate (Sigma-Aldrich).
SEM characterization of histological sections
Unstained bone histological thin sections were coated with 10ânm of carbon and imaged with a Hitachi S-3400N scanning electron microscope with an accelerating voltage of 10âkV. Energy-dispersive X-ray spectroscopy was performed using an Oxford Instruments X-Max detector (20âmm2). Using SEM to characterize histological sections is useful to visualize the in situ mineralization in which the fibrillar collagen matrix disappears in favour of mineral aggregates59,60.
Ewe critical-size defect surgical procedure
Two studies were conducted on ewe. Both studies were reviewed and approved by the IMM Rechercheâs Institutional Animal Care and Use Committee before the initiation of this study. The Animal Care and Use Committee of the IMM Recherche is registered at the CNREEA under the Ethics Committee no. 37. The animal research centre (IMM Recherche) received an agreement (no. 75-14-01) by the Direction Départementale de la Protection des Populations. The studies were also performed in compliance with the Principles of Laboratory Animal Care, formulated by the National Society for Medical Research, and the Guide for the Care and Use of Laboratory Animals, by the Institute for Laboratory Animal Resources (published by the National Academies Press), as amended by the Animal Welfare Act of 1970 (P.L. 91-579) and the 1976 amendments to the Animal Welfare were followed. The surgical procedures were performed as described in ref.â39. This model for bone-defect reconstruction differs from the healing process of larger load-bearing defects, which often necessitate a further specific procedure, such as fixation plate61. A total of eight hole defects (diameter 8âmm, depth 13âmm) per animal (two animals for a total of 16 for ewe study no. 1 and six animals for a total of 48 for ewe study no. 2) were created with a drill into the distal and proximal metaphysis of the humerus and femur. After washing the bone cavity with saline solution, the holes were randomly left empty to heal or fill with the different materials (ewe study no. 1: Col and Col-CHA, nâ=â5 each; ewe study no. 2: ColCG-CHA nâ=â12, VO, MG and AB, nâ=â5 each). Then, the wound was covered with the adjacent tissues and the skin was stapled. Two months after surgery, the sheep were euthanized and both femoral and humeral bones were collected, freed from all overlying tissues and the drill holes were identified. Bone samples were cut perpendicular to the original drill hole.
Faxitron X-ray radiographic imaging
Bone samples were imaged with a cabinet X-ray system to screen, track and evaluate structural, bone density and bone distribution changes using a Faxitron system. Transversal slices of bones taken from the implant sites were submitted to X-ray analysis using Faxitron SR v1.5.
Scanner imaging
Bone samples were submitted to computed tomography scan analysis to investigate 3D reconstruction of the implants sites using a 256 Slice GE Revolution CT Scanner and observed with RadiAnt DICOM Viewer 2022.1.1.
Radiological image analysis
ImageJ software (v1.52a) was used to blindly analyse radiographic images from the rat experiment and Faxitron X-ray and scanner images from the first ewe experiment. First, each image was converted to greyscale and a grey-level threshold was set to discriminate radiopaque and non-radiopaque areas. Then, an area corresponding to the original defect size was set. Pixels with grey level above the threshold, corresponding to radiopaque bone, were counted and divided by the total amount of pixels in the selected area to obtain a bone-filling percentage. For scanner, as full depth acquisition was performed, several relevant images were analysed and the result was expressed as a mean to represent the bone filling of the entire depth of the defect.
Bone histological preparation (ewe)
Samples were fixed in 4% paraformaldehyde solution, dehydrated with increasing ethanol baths and immersed for a week in a solution of butyl methacrylate, methyl benzoate, polyethylene glycol and benzoyl peroxide resin. Polymerization was triggered by the addition of N,N-dimethyl-toluidine and the samples were placed at â20â°C for 48âh. Serial thin sections (4â8âμm) were cut using tungsten carbide knives. Thereafter, the resin was removed using 2-ethoxyethyl acetate and the sections were rehydrated. The sections were then stained for the identification of tissue components in light microscopy analysis, scanned and observed using NDP.view2.8.24.
Histochemical staining
GT (haematoxylin, fuchsine, light green)
The sections were immersed in haematoxylin Weigert solution. After washing, the sections were stained with Ponceau fuchsine. Non-specific staining areas were washed with a 1% phosphomolybdic acid solution. Subsequently, the sections were stained with a light-green solution. After a washing step, the sections were dehydrated with ethanol (increasing gradient) and xylene solutions and then mounted with a resin mounting liquid. Using GT, mineralized bone is stained in green, osteoid in red/orange, nuclei in blue and cytoplasm in light red.
von Kossa/Van Gieson staining
Sections were preincubated with 1% silver nitrate solution under ultraviolet light. Then, the non-specific staining patches were washed with 5% sodium thiosulfate solution. The sections were counterstained with Van Gieson picrofuscine. After a washing step, the sections were dehydrated with ethanol (increasing gradient) and xylene solutions and then mounted with resin mounting fluid. After von Kossa staining, calcium deposits are highlighted in brown-black, osteoid in red and other tissue in yellow.
Haematoxylinâeosin
Sections were immersed in haematoxylin solution. Then, they were rinsed and stained with a 1% eosin solution. After a washing step, the sections were dehydrated with ethanol (increasing gradient) and xylene solutions and then mounted with resin mounting fluid. After HE staining, nuclei are stained purple, cytoplasm pink and collagen pink-red.
Histomorphometry
On the basis of GT and von Kossa-stained histological sections, image analysis (ImageJ2 software v2.0.0) allowed measurement of (1) the amount of mineralized newly formed bone into the defect (mineralized bone surface/total surface) after exclusion of residual biomaterials and (2) the amount of osteoid tissue into the defect (osteoid surface/total surface).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.